
Zinc enzymes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص746-748
الجزء والصفحة:
ص746-748
 2025-10-25
2025-10-25
 52
52
Zinc enzymes
Key point: Zinc is well suited for catalysing acid–base reactions as it is abundant, redox inactive, forms strong bonds to donor groups of amino acid residues, and exogenous ligands such as H2O are exchanged rapidly. A Zn2 ion has high rates of ligand exchange and its polarizing power means that the pKa of a coordinated H2O molecule is quite low. Combined with strong binding to proteinlig ands, rapid ligand exchange (coordinated H2O or substrate molecules), a reasonably high electron affinity, flexibility of coordination geometry, and no complicating redox chemistry, Zn is well suited to its role in catalysing specific acid–base reactions. The large family of Zn enzymes include carbonic anhydrase, carboxypeptidases, alkaline phosphatase, -lactamase (responsible for penicillin resistance in bacteria), and alcohol dehydrogenase. Typically, the Zn is coordinated by three amino acid ligands (in contrast to zinc fingers, which have four) and one exchangeable H2O molecule (14). The mechanisms of Zn enzymes are normally discussed in terms of two limiting cases. In the Zn-hydroxide mechanism, the Zn functions by promoting deprotonation of a bound water molecule, so creating an optimally positioned OH nucleophile that can go on to attack the carbonyl C atom:
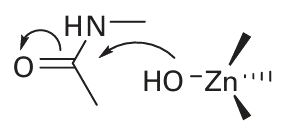
In the Zn-carbonyl mechanism, the Zn ion acts directly as a Lewis acid to accept an electron pair from the carbonyl O atom, and its role is therefore analogous to H in acid catalysis:
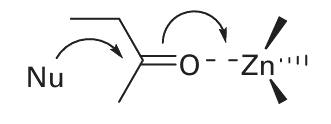
Similar reactions occur with other X O groups, particularly the P O of phosphate esters. There is an obvious advantage of achieving such catalysis at Zn or some other acid species that is anchored in a stereoselective environment. The formation and transport of CO2 is a fundamental process in biology. The solubility of CO2 in water depends on its hydration and deprotonation to form HCO3. However, the uncatalysed reaction at pH=7 is very slow, the forward process occurring with a rate constant of less than 10 3 s 1. Because turnover of CO2 by biological systems is very high, such a rate is far too slow to sustain vigorous aerobic life in a complex organism. In photosynthesis, only CO2 can be used by the enzyme known as ‘rubisco’ (Section 27.9(b)) so rapid dehydration of HCO3 is essential and is one of the first steps in the production of biomass. The CO2 / HCO3 equilibrium is also important (in addition to its role in CO2 transport) because it provides a way of regulating tissue pH.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


