
Other oxygen transport systems
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
740
الجزء والصفحة:
740
 2025-10-23
2025-10-23
 42
42
Other oxygen transport systems
Key point: Arthropods and molluscs use haemocyanin and certain marine worms use haemerythrin. In many organisms, such as arthropods and molluscs, O2 is transported by the Cu protein haemocyanin, which, unlike haemoglobin, is extracellular, as is common for Cu proteins. Haemocyanin is oligomeric, with each monomer containing a pair of Cu atoms in close proximity. Deoxyhaemocyanin (Cu(I)) is colourless but it becomes bright blue when O2 binds. The active site is shown in Fig. 27.19. In the deoxy state, each Cu atom is three-coordinate and bound in a pyramidal array by three histidine residues. The two Cu atoms are so far apart (460 pm) that there is no direct interaction between them. The low coordination number is typical of Cu(I), which is normally two- to four-coordinate. Rapid and reversible coordination of O2 occurs between the two Cu atoms in a bridging dihapto manner (μ-2 2) and the low vibrational wavenumber of the coordinated O2 molecule (750 cm1) shows it has been reduced to peroxide O2-2, with an accompanying lowering of the bond order from 2 to 1. To accommodate the binding of O2, the protein adjusts its conformation to bring the two Cu atoms closer together. The Cu sites become five-coordinate, which is typical of Cu (II). Haemerythrin is an example of a special class of dinuclear Fe centres that are found in a number of proteins with diverse functions, such as methane monooxygenase, and some ribonucleotide reductases and acid phosphatases. The two Fe atoms in the active site of haemerythrin (21) are each coordinated by amino acid side chains but are also linked by two bridging carboxylate groups and a small ligand. In the reduced form, which binds O2 reversibly, this small ligand is an OH- ion. Coordination of O2 occurs at only one of the Fe atoms and the distal O atom forms a hydrogen bond to the H atom of the bridging hydroxide.
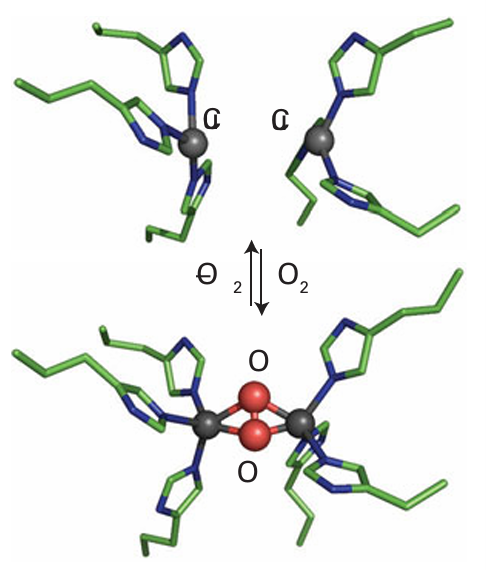
Figure 27.19 Binding of O2 at the active site of haemocyanin causes the two Cu atoms to be brought closer together. The O2 complex is regarded as a binuclear Cu(II) centre in which the two Cu atoms are bridged by an n2, n2-peroxide.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


