
Iron–sulfur clusters
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص744-745
الجزء والصفحة:
ص744-745
 2025-10-23
2025-10-23
 43
43
Iron–sulfur clusters
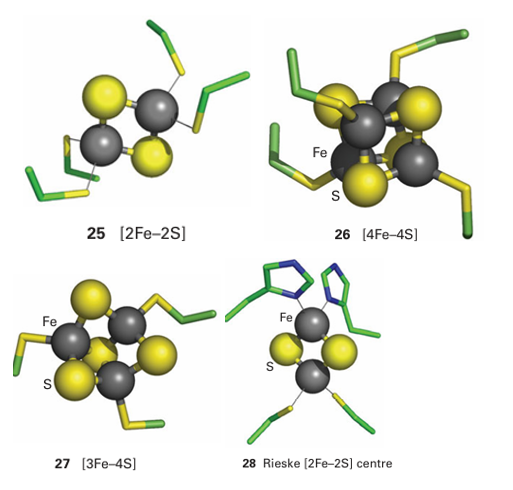
Key points: Iron–sulfur clusters generally operate at more negative potentials than cytochromes; they are composed of high-spin Fe (III) or Fe (II) with sulfur ligands in a mainly tetrahedral environment. Iron–sulfur clusters are widespread in biology, although their importance was not established as early as cytochromes on account of their lack of distinctive optical characteristics. By convention, FeS clusters are represented by square brackets showing how many Fe and non-protein S atoms are present, as in [2Fe–2S] (25), [4Fe–4S] (26), and [3Fe–4S] (27). The efficacy of FeS clusters as fast ET centres is largely due to their being able to delocalize the added electron to varying degrees, which minimizes bond length changes and decreases the reorganization energy. The presence of sulfur ligands to provide good lead-in groups is also important. Small ET proteins containing FeS clusters are known as ferredoxins, where as in many large enzymes, FeS clusters are arranged in a relay, less than 1.5 nm apart, to link remote redox sites in the same molecule. The relay concept is illustrated in Fig. 27.26 with a class of enzymes known as hydrogenases, which we discuss further in Section 27.14. In nearly all cases, the Fe atoms are tetrahedrally coordinated by cysteine thiolate (RS-) groups as the protein ligands. The overall assembly, including the protein ligands, is known as an ‘FeS centre’. Examples are known in which one or more of the Fe atoms is coordinated by non-thiolate amino acid ligands, such as carboxylate, imidazole, and alkoxyl (serine), or by an exogenous ligand such as H2 O or OH, and the coordination number about the Fe subsite may be increased to six. The cubane [4Fe 4S] (26) and cuboidal [3Fe 4S] (27) clusters are obviously closely related, and may even interconvert within a protein by the addition or removal of Fe from one subsite. Larger clusters also occur, such as the ‘super clusters’ [8Fe 7S] and [Mo–7Fe–8S–X] found in nitrogenase (Section 27.13). Despite the presence of more than one Fe atom, FeS clusters generally carry out single electron transfers and are good examples of mixed-valence systems comprising Fe (III) and Fe (II). The redox state of a cluster is commonly represented by summing the charges due to Fe (3+or 2+, respectively) and S atoms (2-) and the resultant overall charge, which is referred to as the oxidation level, is written as a superscript.
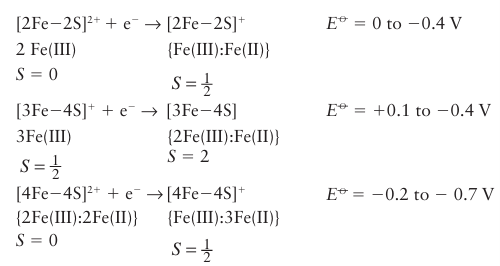
Most FeS centres have negative reduction potentials (usually more negative than −0.2 V) so the reduced forms are good reducing agents: exceptions are [4Fe-4S] clusters that operate instead between the +3 and +2 oxidation states (these are called ‘HiPIP’ centres because they were originally discovered in a protein called high-potential iron protein for which the reduction potential is 0.35 V) and so-called Rieske centres, which are [2Fe-2S] clusters having one Fe subsite coordinated by two neutral imidazole ligands rather than cysteine (28). Coordination by histidine stabilizes the iron as Fe (II) and usually raises the reduction potential to a much more positive value (above 0.2 V). The half-reactions have been written to include the spin states of FeS clusters: individual Fe atoms are high spin, as expected for tetrahedral coordination by S2-, and different magnetic states arise from ferromagnetic and antiferromagnetic coupling (Section 20.8). These magnetic properties are very important, as they allow the centres to be investigated by EPR (Section 8.6). A major question is how FeS centres are synthesized and inserted into the protein. This process has been studied mostly in prokaryotes, from which it is known that specific proteins are involved in the supply and transport of Fe and S atoms, their assembly into clusters, and their transfer to target proteins. Free sulfide (H2S, HS-, or S2-) in a cell is highly poisonous, so it is produced only when required by an enzyme called cysteine desulfurase, which breaks down cysteine to yield S2- ions and alanine.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


