
Iron enzymes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص750-751
الجزء والصفحة:
ص750-751
 2025-10-25
2025-10-25
 53
53
Iron enzymes
Key points: Acid phosphatases contain a dinuclear metal site containing Fe (III) in conjunction with Fe, Zn, or Mn; aconitase contains a [4Fe–4S] cluster, one subsite of which is modified to manipulate the substrates.
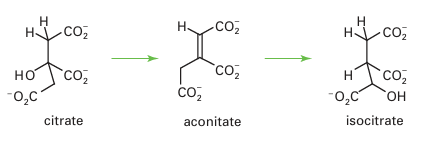
Acid phosphatases, sometimes known as ‘purple’ acid phosphatases (PAPs) on account of their intense colour, occur in various mammalian organs, particularly the bovine spleen and porcine uterus. Acid phosphatases catalyse hydrolysis of phosphate esters, with op-timal activity under mild acid conditions. They are involved in bone maintenance and hydrolysis of phosphorylated proteins (therefore they are important in signalling). They may also have other functions, such as Fe transport. The pink or purple colours of acid phosphatases are due to a tyrosinate → Fe (III) charge-transfer transition at 510–550 nm (ε=4000 dm3 mol–1 cm–1). The active site contains two Fe atoms linked by ligands, similar to haemerythrin (21). Acid phosphatases are inactive in the oxidized {Fe (III)Fe (III)} state in which they are often isolated. In the active state, one Fe is reduced to Fe (II). Both Fe atoms are high spin and remain so throughout the various stages of reactions. Acid phosphatases also occur in plants, and in these enzymes the reducible Fe is replaced by Zn or Mn. The active site of an acid phosphatase from sweet potato (33) shows how phosphate becomes coordinated to both Fe (III) and Mn (II) ions. In the mechanism shown in Fig. 27.32, rapid binding of the phosphate group of the ester occurs to the M(II) sub site, then the P atom is attacked by an OH– ion that is formed at the more acidic Fe (III) subsite. The FeZn centre is also found in an important enzyme called calcineurin, which catalyses the phosphorylation of serine or threonine residues on certain protein surfaces, in particular a transcription factor involved in controlling the immune response. Calcineurin is activated by Ca2 binding, both directly and through calmodulin. Aconitase is an essential enzyme of the tricarboxylic acid cycle (also known as the Krebs cycle, or citric acid cycle), the main source of energy production in higher organisms, where it catalyses the interconversion of citrate and isocitrate in a reaction that formally involves dehydration and rehydration, and proceeds through an intermediate, aconitate, which is released in small amounts:
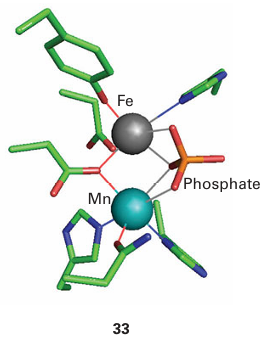
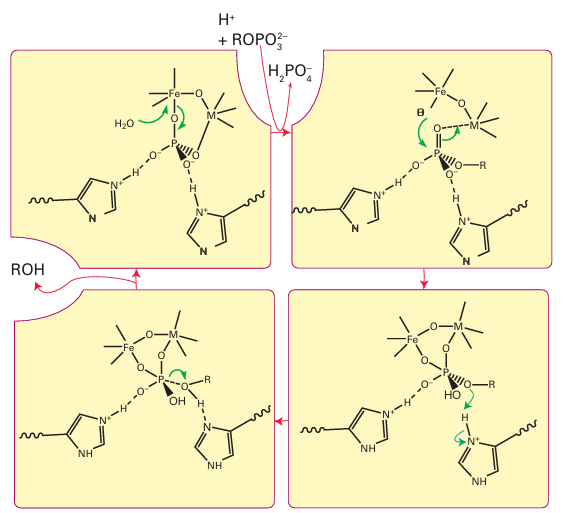
Figure 27.32 Proposed mechanism of action of acid phosphatase. The metal site M(II) is occupied by Fe (most common in animals) or by Mn or Zn (in plants).
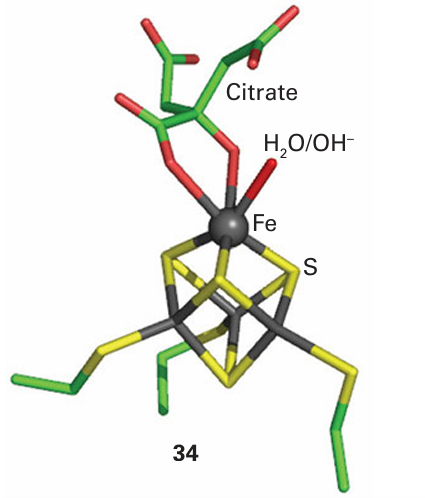
The active form of the enzyme contains a [4Fe–4S] cluster, which degrades to [3Fe–4S] when the enzyme is exposed to air. The site of catalysis is the Fe atom that is lost on oxida tion. The structure shows that this unique subsite is not coordinated by a protein ligand but by an H2 O molecule, which explains why this Fe is more readily removed. A plausible mechanism for the action of aconitase, based on structural, kinetic, and spectroscopic evidence, involves the binding of citrate to the active Fe subsite, which increases its coordination number to 6. An intermediate in the catalytic cycle is ‘captured’ for X-ray diffraction investigation, using a site-directed mutant that can bind the citrate but cannot complete the reaction (34). The Fe atom polarizes a C–O bond and OH is abstracted, while a nearby base accepts a proton. The substrate now swings round, and the OH and H are reinserted onto different positions. A form of aconitase that is found in cytoplasm has another intriguing role, that of an Fe sensor (Section 27.15).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


