


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-1-2017
Date: 8-3-2019
Date: 10-3-2017
|
Cyanogen and its derivatives
The CN- radical is a pseudo-halogen, i.e. its chemistry resembles that of a halogen atom, X; it forms C2N2, HCN and [CN]-, analogues of X2, HX and X-. Although C2N2 and HCN are thermodynamically unstable with respect to decomposition into their elements, hydrolysis by H2O, and oxidation by O2, they and [CN]- are kinetically stable enough for them to be well-established and much studied species. Cyanogen, C2N2, is a toxic, extremely flammable gas (mp 245 K, bp 252 K) which is liable to react explosively with some powerful oxidants. Although ΔfHo(C2N2, 298K) = 297 kJ mol-1, pure C2N2 can be stored for long periods without decomposition. the tow reactions that showen in below equation give two syntheses of C2N2; below reaction illustrates the pseudo-halide like nature of [CN]- which is oxidized by Cu(II) in an analogous fashion to the oxidation of I- to I2. Cyanogen is manufactured by air-oxidation of HCN over a silver catalyst.
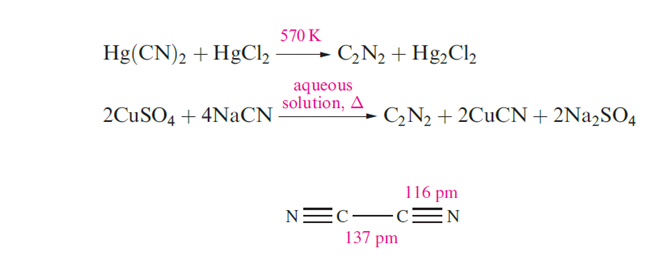
Cyanogen has the linear structure and the short C_C distance indicates considerable electron delocalization. It burns in air with a very hot, violet flame and resembles the halogens in that it is hydrolyzed by alkali and undergoes thermal dissociation to CN- at high temperatures.
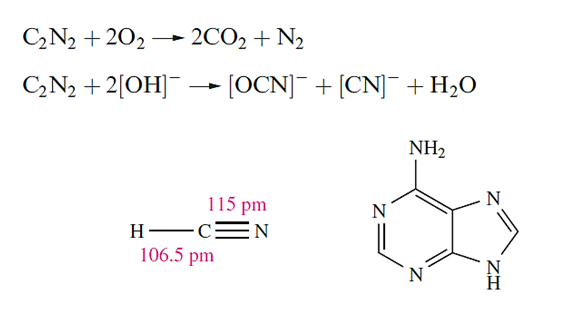
Hydrogen cyanide, HCN is an extremely toxic and flammable, colourless volatile liquid (mp 260 K, bp 299 K) with a high dielectric constant due to strong hydrogen bonding; it has a characteristic smell of bitter almonds. The pure liquid polymerizes to HC(NH2)(CN)2 and (H2N)(NC)C=C(CN)(NH2) mixed with higher molecular mass polymers, and in the absence of a stabilizer such as H3PO4, polymerization may be explosive. In the presence of traces of H2O and NH3, HCN forms adenine and on reduction, gives MeNH2. It is thought that HCN was one of the small molecules in the early atmosphere of the Earth, and played an important role in the formation of many biologically important compounds. Hydrogen cyanide is prepared on a small scale by adding acid to NaCN, and industrially.
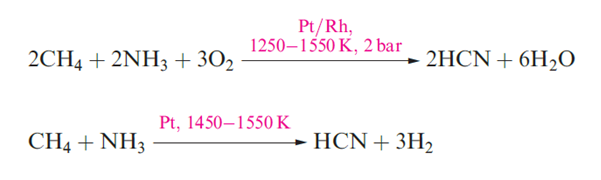
Many organic syntheses involve HCN, and it is of great industrial importance, a large fraction going into the production of 1,4-dicyanobutane (adiponitrile) for nylon manufacture, and cyanoethene (acrylonitrile) for production of acrylic fibres. In aqueous solution, HCN behaves as a weak acid (pKa = 9.31) and is slowly hydrolysed. An older name for hydrocyanic acid is prussic acid.
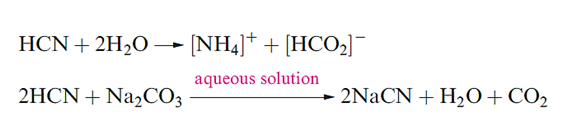
The neutralization of aqueous HCN by Na2CO3, NaHCO3 or Na[HCO2] generates NaCN, the most important salt of the acid. It is manufactured by reaction as in above and it has widespread uses in organic chemistry (e.g. for the formation of C_C bonds); it is also used in the extraction of Ag and Au.
At 298 K, NaCN and KCN adopt the NaCl lattice, each [CN]- ion freely rotating (or having random orientations) about a fixed point in the lattice and having an effective ionic radius of ≈190 pm. At lower temperatures, transitions to structures of lower symmetry occur, e.g. NaCN undergoes a cubic to hexagonal transition below 283 K. Crystals of NaCN and KCN are deliquescent, and both salts are soluble in water and are highly toxic. Fusion of KCN and sulfur gives potassium thiocyanate, KSCN. Mild oxidizing agents convert [CN]- to cyanogen but with more powerful oxidants such as PbO or neutral [MnO4]-, cyanate ion is formed. Potassium cyanate reverts to the cyanide on heating .
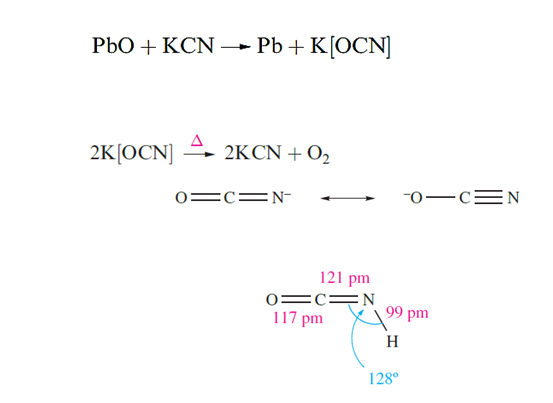
Two acids can be derived from HOCN (cyanic acid or hydrogen cyanate) and HNCO (isocyanic acid). It has been established that HOCN and HNCO are not in equilibrium with each other. Isocyanic acid (pKa = 3.66) is obtained by heating urea but rapidly trimerizes, although heating the trimer regenerates the monomer.
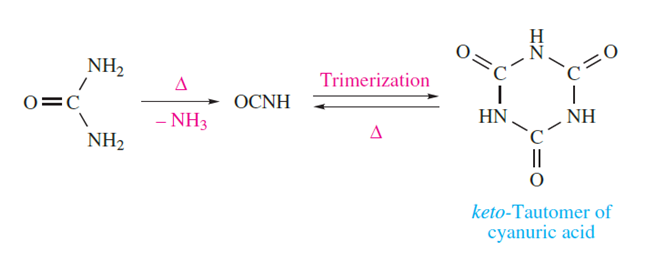
The fulminate ion, [CNO] -, is an isomer of the cyanate ion. Fulminate salts can be reduced to cyanides but cannot be prepared by oxidation of them. The free acid readily polymerizes but is stable for short periods in Et2O at low temperature. Metal fulminates are highly explosive; mercury(II) fulminate may be prepared and is a dangerous detonator.
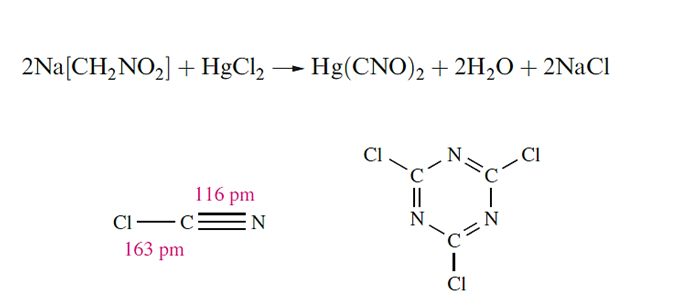
Cyanogen chloride (mp 266 K, bp 286 K), is prepared by the reaction of Cl2 with NaCN or HCN, and readily trimerizes which has applications in the manufacture of dyestuffs and herbicides.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|