

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Thermodynamic considerations of complex formation
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 182
10-3-2017
3705
Thermodynamic considerations of complex formation
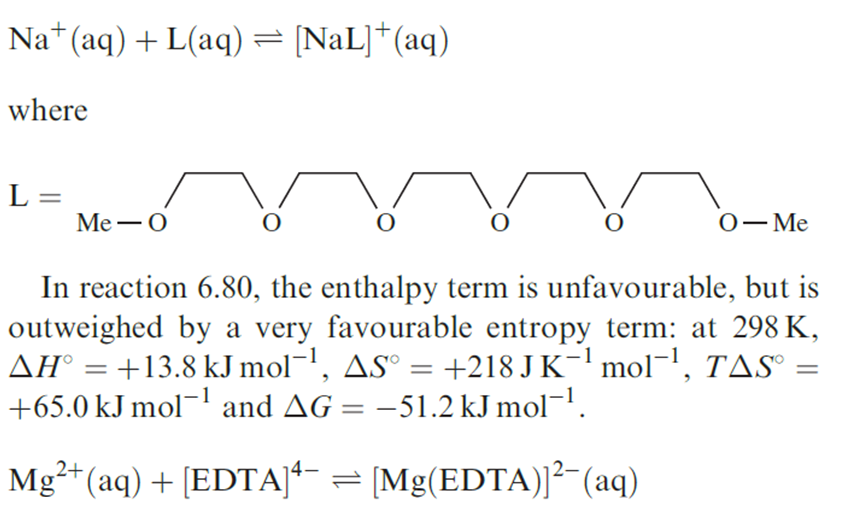
A detailed discussion of the thermodynamics of complex in aqueous solution lies beyond the scope of this book, but we discuss briefly entropy changes that accompany the formation of coordination compounds in solution, and the so-called chelate effect.
We saw in Section 6.9 that highly charged ions have more negative values of ΔhydSo than singly charged ions, and this can be viewed in terms of the highly charged ions imposing more order on H2O molecules in the environment of the ion.
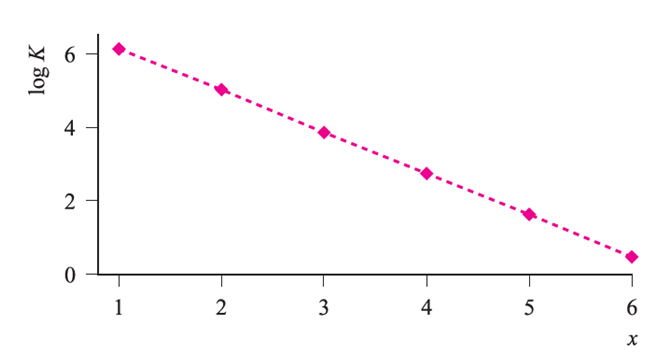
Fig. 1.1 Stepwise stability constants for the formation of [Al(H2O)6-xFx](3-x)+ (x = 1–6).
When complex formation occurs between highly charged cations and anions, with a resulting partial or total cancellation of charges, the changes in enthalpy for these processes are significantly negative. However, the accompanying changes in entropy are significantly positive because less order is imposed on the H2O molecules around the complex ion than around the uncomplexed, metal cations and anionic ligands. The corresponding values of ΔGo are, therefore, substantially negative indicating that very stable complexes are formed. For example, ΔSo(298 K) for reaction 1.1 is 117 JK-1 mol-1 and ΔGo(298 K) is _60.5 kJ mol-1; the ligand in equation 1.1 is [EDTA]4-.
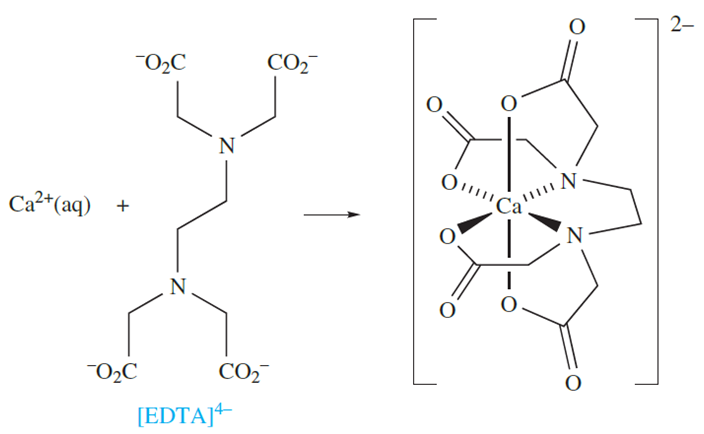
(1.1)
Another source of increase in entropy is important: when we are dealing with comparable uncharged ligands (e.g. NH3 and H2NCH2CH2NH2), polydentate ligands form more stable complexes than monodentate ones.
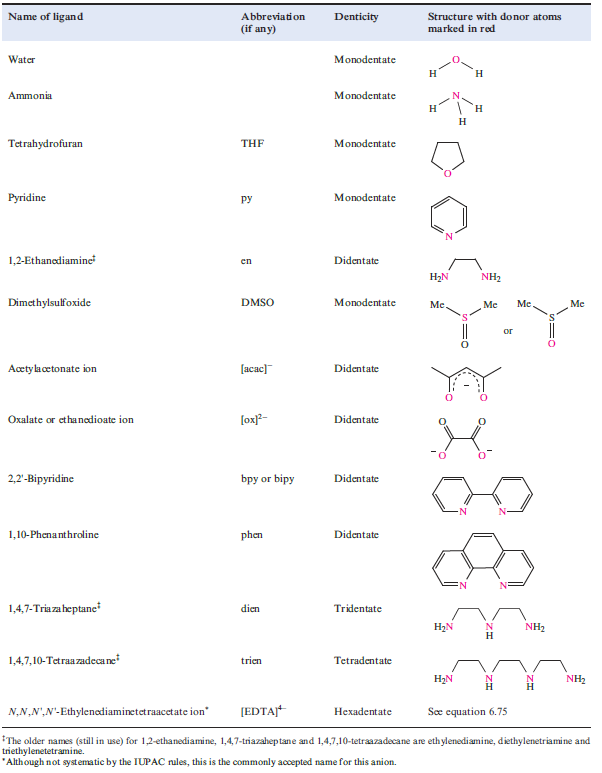
The number of donor atoms through which a ligand coordinates to a metal ion is defined as the denticity of the ligand; a monodentate ligand possesses one donor atom (e.g. NH3), a didentate‡ ligand two (e.g. [acac]-) and so on. In general, a ligand with more than one donor atom is termed polydentate.
Coordination of a polydentate ligand to an ion leads to the formation of a chelate ring, and five such rings can be seen in [Ca(EDTA)]-in equation 1.1. The word chelate is derived from the Greek for a crab’s claw. Table 1.1 lists some common ligands; en, [ox]-and bpy form 5-membered chelate rings on coordination to a metal ion, whereas coordination of [acac]- gives a 6-membered ring (Figure 6.10). Both 5- and 6-membered chelate rings are common in metal complexes. Each ring is characterized by a bite angle, i.e. the X-M-Y angle where X and Y are the two donor atoms of the chelating ligand (structure 1.1). Ringstrain causes the formation of 3- and 4-membered rings to be relatively unfavourable.
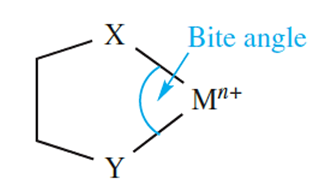
(1.1)
The 6-membered ring formed when [acac] - chelates to a metal ion (Figure 6.10) is planar and is stabilized by delocalized π-bonding. Ligands such as bpy and [ox]- also produce planar chelate rings upon interaction with a metal centre. A saturated diamine such as en (1.2) is more flexible and adopts a puckered ring as is shown in Figure 1.2 for a general [M(en)3]n+ complex. Adding one more carbon atom to the backbone of the ligand en gives 1,3-propanediamine (pn, 1.3).
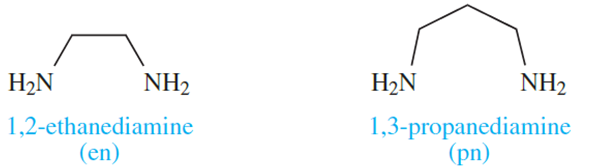
(1.2) (1.3)
For flexible, saturated N-donor ligands of this type, experimental data reveal that small metal ions favour ligands that form 6-membered chelate rings, whereas larger metal ions favour ligands that give 5-membered chelate rings. A general conclusion that ‘5-membered rings are more stable than 6-membered chelate rings’ is often cited in textbooks.
Table 1.1 Names and structures of selected ligands.
However, this statement needs to be qualified, taking into account the size of the metal ion. The enhanced complex stability observed when a small metal ion resides within a 6-membered rather than a 5-membered chelate ring (the ligand being a saturated one such as a diamine) has been explained in terms of a model in which the metal ion replaces an sp3 hybridized C atom in cyclohexane. For this replacement to be optimized, the bite angle (1.1) should be close to 109.58 (i.e. the angle for a tetrahedral C atom), and the M_N bond length should be 160 pm. When diamines coordinate to larger metal ions (e.g. Pb2+, Fe2+, Co2+), the most stable complexes tend to be those involving ligands that form 5-membered chelate rings. The ideal parameters are a bite angle of 698 and an M_N bond
length of 250 pm.*
We now compare the stability of complexes formed between a given metal ion and related monodentate and didentate ligands, and address the so-called chelate effect.
In order to make meaningful comparisons, it is important to choose appropriate ligands. An NH3 molecule is an approximate (but not perfect) model for half of the ligand en. Equations below show equilibria for the displacement of pairs of NH3 ligands in [Ni(H2O)6_2n(NH3)2n]2+ (n = 1, 2 or 3) by en ligands. The logK and ΔG0 values refer to the equilibria at 298 K.
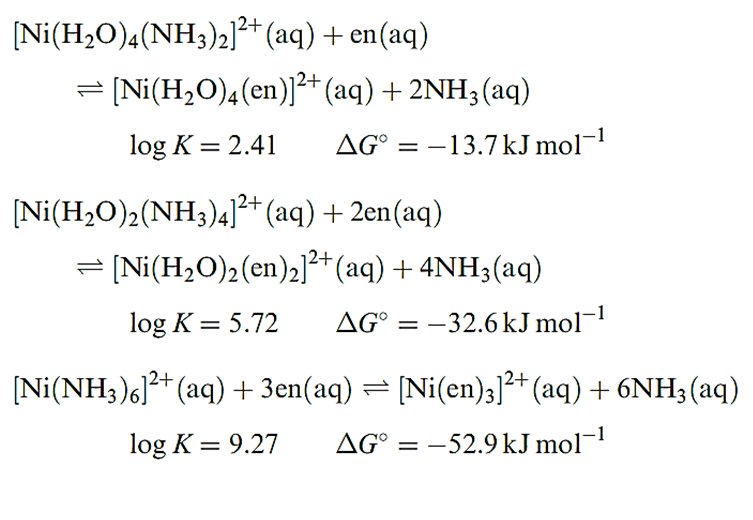
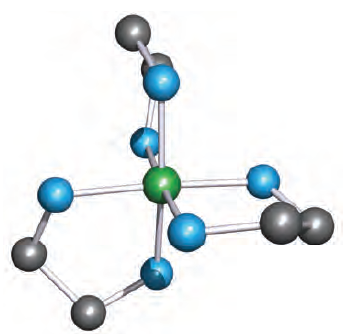
Fig. 1.2 This modelled structure of a complex [M(en)3]n illustrates that the ligand en coordinates to give a puckered chelate ring. Colour code: M, green; N, blue; C, grey.
For each ligand displacement, ΔG0 is negative and these data (or the values of log K) illustrate that the formation of each chelated complex is thermodynamically more favourable than the formation of the corresponding ammine complex. This phenomenon is called the chelate effect and is a general observation.
For a given metal ion, the thermodynamic stability of a chelated complex involving didentate or polydentate ligands is greater than that of a complex containing a corresponding number of comparable monodentate ligands. This is called the chelate effect. The value of ΔG0 for a reaction such as 1.18 gives a measure of the chelate effect and from the equation
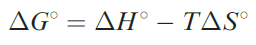
we can see that the relative signs and magnitudes of the contributing ΔH0and T ΔS0 terms are critical.† For reaction 1.18 at 298 K, ΔH0 = _16.8 kJ mol-1 and ΔS0 = 121 JK-1 mol-1 ; the T ΔS0 term is +36.1 kJ mol-1 . Thus, both the negative ΔH0and positive T ΔS0 terms contribute to the overall negative value of ΔG0 . In this particular case, the T ΔS0 term is larger than the ΔH0 term. However, the mutual reinforcement of these two terms is not a general observation as the following examples illustrate. For reaction 1.19, ΔG0 (298 K)= _8.2 kJ mol-1. This favourable energy term arises from entropy and enthalpy contributions of T ΔS0= _8.8 kJK_1 mol_1 and ΔH0 = _17.0 kJ mol-1, i.e. a favourable enthalpy term tha more than compensates for the unfavourable entropy term.
In order to examine the origins of the enthalpy and entropy contributions, we again consider reaction 1.18. It has been suggested that the enthalpy contribution to the chelate effect arises from several effects:
- a reduction in the electrostatic repulsion between the δ donor atoms (or negatively charged donor atoms in the case of some ligands) on going from two monodentate ligands to one didentate ligand;
- desolvation effects involving the disruption of ligand- H2O hydrogen-bonded interactions upon complex formation – such hydrogen-bonded interactions will be greater for, for example, NH3 than for en;
- an inductive effect of the CH2CH2 bridges in didentate or polydentate ligands which increases the donor strength of the ligand with respect to a corresponding monodentate ligand, e.g. en versus NH3.
The entropy contribution to the chelate effect is easier to visualize. In equations 6.81 and 6.82, two comparable reactions are shown.
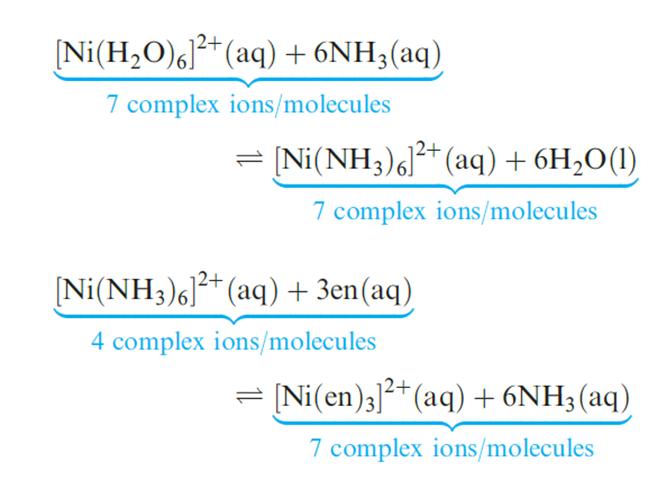
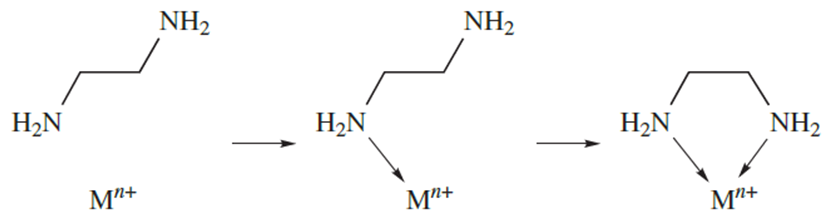
In reaction 6.81, monodentate ligands are involved on both sides of the equation, and there is no change in the number of molecules or complex ions on going from reactants to products. However, in reaction below which involves didentate ligands replacing monodentate ligands, the number of species in solution increases on going from reactants to products and there is a corresponding increase in entropy (ΔS is positive). Another way of looking at the entropy effect is illustrated in diagram 1.4. In forming a chelate ring, the probability of the metal ion attaching to the second donor atom is high because the ligand is already anchored to the metal centre. In contrast, the probability of the metal ion associating with a second monodentate ligand is much lower.
Entropy effects associated with desolvation of the ligands prior to complex formation also play a role. So far, we have considered only the coordination of monodentate or acyclic polydentate ligands. A wealth of coordination chemistry involves macrocyclic ligands, which include the family of crown ethers (for example, 18-crown-6, 1.5, and benzo-12-crown-4, 1.6), and the encapsulating cryptand ligands.
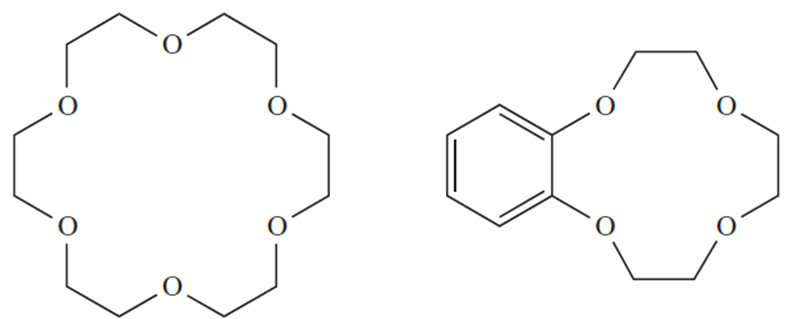
(1.5) (1.6)
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















