


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-8-2017
Date: 18-9-2017
Date: 31-8-2017
|
NITRATION OF TOLUENE
Nitration of toluene is the only important reaction that involves the aromatic ring rather than the aliphatic methyl group. The nitration reaction occurs with an electrophilic substitution by the nitronium ion. The reaction conditions are milder than those for benzene due to the activation of the ring by the methyl substituent. A mixture of nitrotoluenes results. The two important monosubstituted nitrotoluenes are o- and p-nitrotoluenes:
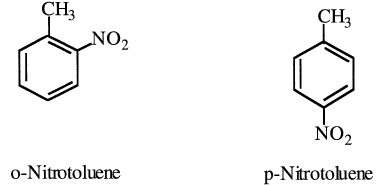
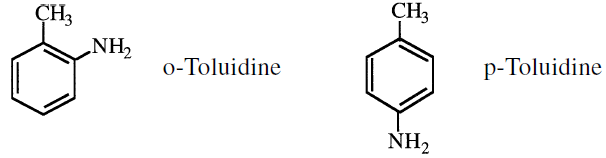
Mononitrotoluenes are usually reduced to corresponding toluidines, which make dyes and rubber chemicals:
Dinitrotoluenes are produced by nitration of toluene with a mixture of concentrated nitric and sulfuric acid at approximately 80°C. The main products are 2,4- and 2,6-dinitrotoluenes:
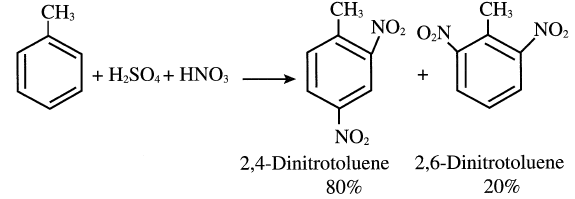
The dinitrotoluenes are important precursors for toluene diisocyanates (TDI), monomers used to produce polyurethanes. The TDI mixture is synthesized from dinitrotoluenes by a first-step hydrogenation to the corresponding diamines. The diamines are then treated with phosgene to form TDI. The yield from toluene is approximately 85%:
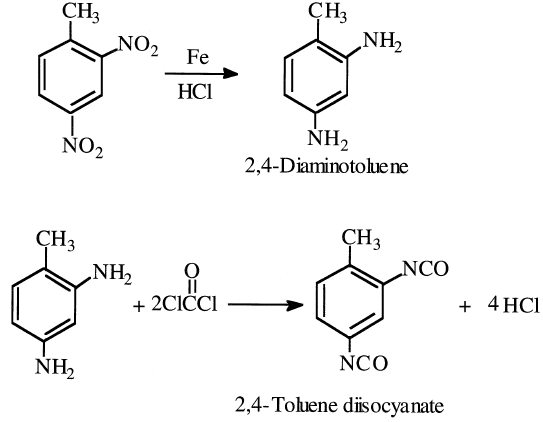
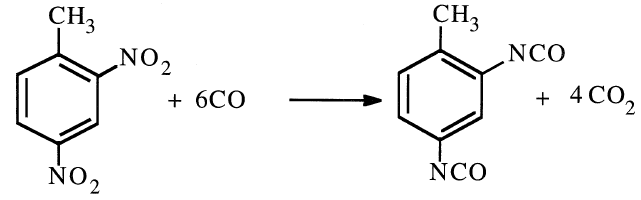
An alternative route for TDI is through a liquid-phase carbonylation of dinitrotoluene in presence of PdCl2 catalyst at approximately 250°C and 200 atmospheres:
Trinitrotoluene TNT is a well-known explosive obtained by further nitration of the dinitrotoluenes.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|