


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-7-2017
Date: 16-7-2017
Date: 1-11-2020
|
Entropy and the Second Law of Thermodynamics
When NH4Cl goes into solution, the NH4+ and Cl- ions leave the highly ordered environment of the crystal to enter the chaotic world of a solution. This increase in disorder is the driving force for the endothermic dissolution of NH4Cl.
The state function which measures disorder is the entropy, S, and the second law of thermodynamics may be stated as follows: The entropy of the universe or of an isolated system always increases when a spontaneous irreversible process occurs; entropy remains constant in a reversible process, i.e., a process which remains at equilibrium for every step along the way,
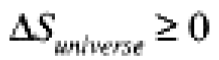 (1.1)
(1.1)
The distinction between reversible and irreversible processes is illustrated by the following example.
Example
Consider a mixture of liquid and solid benzene at its normal freezing point, 5.45°C. If the temperature is raised by a tiny amount, say to 5.46°C, the solid portion will gradually melt; if the temperature were decreased to 5.44°C, the liquid would gradually crystallize. Freezing and melting are reversible processes at 5.45°C.
It is possible to cool liquid benzene to a temperature below the normal freezing point, say to 2°C, without crystallization. The liquid is then said to be supercooled. If a tiny crystal of solid benzene is added, the liquid will crystallize spontaneously and irreversibly. Raising the temperature to 2.01°C (or even to 3°C) will not stop the crystallization. One would have to raise the temperature to above 5.45°C to restore the liquid state. The crystallization of liquid benzene at 2.00°C is an example of an irreversible process. For a reversible process, the change in a system's entropy is:
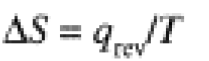 (1.2)
(1.2)
Since entropy is a state function, we can use a reversible process with the same initial and final states to calculate the entropy change that occurs in an irreversible process. Eq. (1.2) gives us a criterion for spontaneity, but it is restricted to systems that are not in thermal or mechanical contact with the surroundings. A more useful criterion is based on the Gibbs free energy, G, defined by
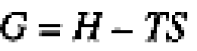 (1.3)
(1.3)
Equation (1.2) leads to the following criterion for spontaneity for a process occurring at constant temperature and pressure, but with the system in thermal and mechanical contact with the surroundings: The Gibbs free energy decreases for a spontaneous (irreversible) process and remains constant for an equilibrium (reversible) process.
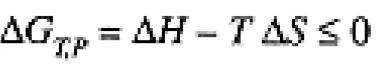 (1.4)
(1.4)
G decreases for a spontaneous process, like the energy of a mechanical system. Since ΔG incorporates both driving forces for spontaneity—enthalpy (energy) decrease and entropy (disorder) increase—an endothermic process may be spontaneous if the increase in disorder is big enough to counteract the unfavorable enthalpy change, and a process that leads to increased order (negative ΔS) may be spontaneous if the process is sufficiently exothermic (negative ΔH).



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|