


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-2-2016
Date: 19-2-2019
Date: 13-4-2019
|
Nomenclature Based on Oxidation States
Many metals exhibit two or more oxidation states. This is particularly true of the transition metals, where cations with ONs of +2 and +3 are common, but also of the heavier main-group metals such as thallium (ONs +1 and +3), and tin and lead (ONs of +2 and +4).
The modern system of nomenclature uses ONs directly in naming compounds. Thus in FeO and Fe2O3, the ONs of Fe are +2 and +3, respectively, and we name these compounds iron(II) oxide and iron(III) oxide. Similarly, phosphorus(III) cholride is PCl3 and phosphorus(V) chloride is PCl5.
The traditional nomenclature for salts of such metals is to use the suffix -ic for the higher ON and –ous for the lower, often using the Latin name for the element, e.g., ferrous (Fe2+) and ferric (Fe3+), stannous (Sn2+) and stannic (Sn4+), chromous (Cr2+) and chromic (Cr3+).
Monatomic and polyatomic anions containing a single element are named with the -ide suffix, regardless of the formal oxidation state, e.g., chloride (Cl-), sulfide (S2-), and triiodide (I3-).
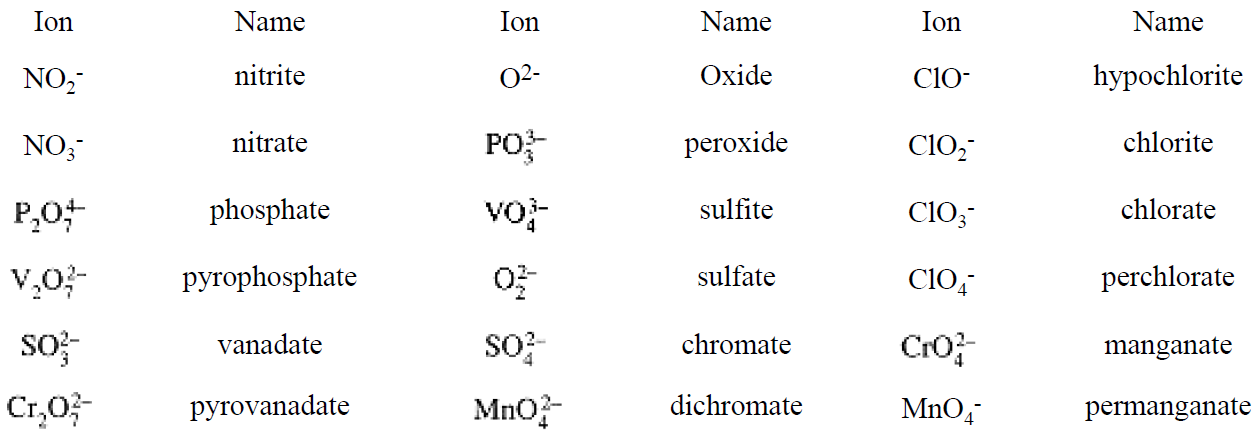
Both metals and nonmetals form oxoanions with the central atom in a variety of oxidation states, e.g., NO2- and NO3-, MnO42- and MnO4-. The traditional names reflect these differences by using the suffix - ate for the higher oxidation number and -ite for the lower. When more than two oxidation states are found, the highest uses the -ate suffix with the prefix per-, and the lowest uses the -ite suffix with the prefix hypo-. Some oxoanions with two central atoms linked by an oxygen use the di- prefix (indicating 2), others use the pyro- prefix, (indicating their origins as products obtained by heating—pyrolysis).
Some of the more common polyatomic anions are listed in Table 1.1.
Table 1.1. Common Anions Exhibiting Variable ON's



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|