


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 22-5-2016
Date: 22-5-2016
Date: 27-3-2021
|
Energy Quanta
One experiment that demonstrates an inconsistency between experimental results and the classical theory of light is called the photoelectric effect. If monochromatic light is incident on a clean surface of a material, then under certain conditions, electrons (photoelectrons) are emitted from the surface. According to classical physics. if the intensity of the light is large enough, the work function of the material will be overcome and an electron will be emitted from the surface independent of the incident frequency. This result is not observed. The observed effect is that, at a constant incident intensity, the maximum kinetic energy of the photoelectron varies linearly with frequency with a limiting frequency v = v0. below which no photoelectron is produced. This result is shown in Figure 1.1. If the incident intensity varies at a constant frequency, the rate of photoelectron emission changes, but the maximum kinetic energy remains the same.
Planck postulated in I900 that thermal radiation is emitted from a heated surface in discrete packets of energy called quanta. The energy of these quanta is given by E = hv, where v is the frequency of the radiation and h is a constant now known as Planck's constant (h = 6.625 × l0-34 J-s). Then in 1905. Einstein interpreted the photoelectric results by suggesting that the energy in a light wave is also contained in discrete packets or bundles. The particle-like packet of energy is called a photon, whose energy is also given by E = hv. A photon with sufficient energy, then, can knock an electron from the surface of the material. The minimum energy required to remove an electron is called the work function of the material
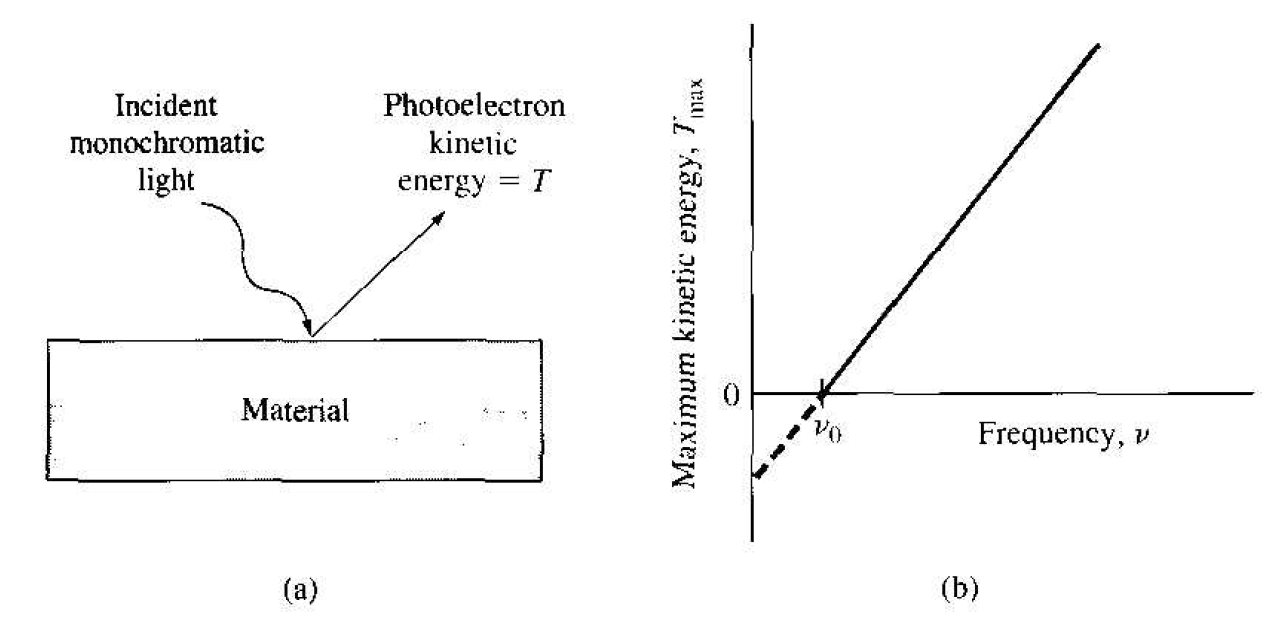
Figure 1.1 (a) The photoelectric effect and (b) the maximum kinetic energy of the photoelectron as a function of incident frequency.
and any excess photon energy goes into the kinetic energy of the photoelectron. This result was confirmed experimentally as demonstrated in Figure 1.1. The photoelectric effect shows the discrete nature of the photon and demonstrates the particle-like behavior of the photon.
The maximum kinetic energy of the photoelectron can be written as
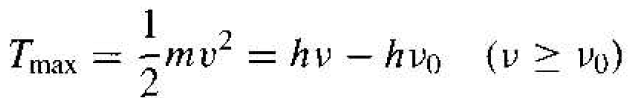 (1)
(1)
where hv is the incident photon energy and hv0 is the minimum energy, of work function, required to remove an electron from the surface.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|