


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-12-2019
Date: 13-12-2019
Date: 25-7-2018
|
A Simple Expression Relates pH, pKa, and Buffer Concentration
The titration curves of acetic acid, H2PO4- , and NH4- have nearly identical shapes, suggesting that these curves reflect a fundamental law or relationship. This is indeed the case. The shape of the titration curve of any weak acid is described by the Henderson- Hasselbalch equation, which is important for understanding buffer action and acid-base balance in the blood and tissues of vertebrates.
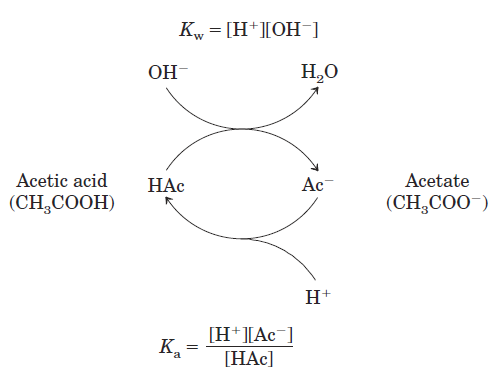
FIGURE 1.1 The acetic acid–acetate pair as a buffer system. The system is capable of absorbing either H+ or OHˉ through the reversibility of the dissociation of acetic acid. The proton donor, acetic acid (HAc), contains a reserve of bound H+, which can be released to neutralize an addition of OHˉ to the system, forming H2O. This happens because the product [H+][OHˉ] transiently exceeds Kw (1×10ˉ14 M2). The equilibrium quickly adjusts so that this product equals 1 × 10ˉ14 M2 (at 25 oC), thus transiently reducing the concentration of H+. But now the quotient [H+][Acˉ] / [HAc] is less than Ka, so HAc dissociates further to restore equilibrium. Similarly, the conjugate base, Acˉ, can react with H+ ions added to the system; again, the two ionization reactions simultaneously come to equilibrium. Thus a conjugate acid-base pair, such as acetic acid and acetate ion, tends to resist a change in pH when small amounts of acid or base are added. Buffering action is simply the consequence of two reversible reactions taking place simultaneously and reaching their points of equilibrium as governed by their equilibrium constants, KW and Ka.
This equation is simply a useful way of restating the expression for the dissociation constant of an acid. For the dissociation of a weak acid HA into H+ and Aˉ, the Henderson-Hasselbalch equation can be derived as follows:
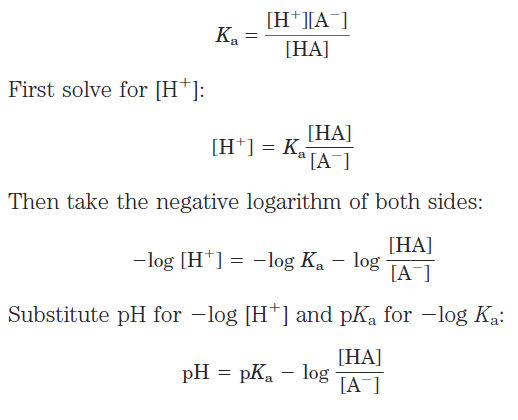
Now invert _log [HA]/[Aˉ], which involves changing its sign, to obtain the Henderson-Hasselbalch equation:
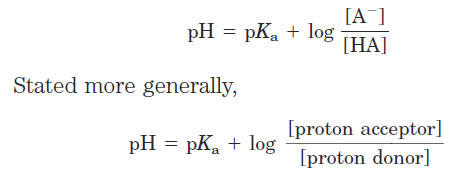
This equation fits the titration curve of all weak acids and enables us to deduce a number of important quantitative relationships. For example, it shows why the pKa of a weak acid is equal to the pH of the solution at the midpoint of its titration. At that point, [HA] equals [Aˉ], and

the ratio of proton donor and acceptor; (2) calculate pH, given pKa and the molar ratio of proton donor and acceptor; and (3) calculate the molar ratio of proton donor and acceptor, given pH and pKa.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|