


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-11-2020
Date: 26-2-2019
Date: 15-6-2019
|
Tetraselenium tetranitride
Among the compounds formed by Se and N, we mention only Se analogues of S4N4. Selenium tetranitride, Se4N4, can be prepared by reacting SeCl4 with {(Me3Si)2N}2Se. It forms orange, hygroscopic crystals and is highly explosive. The structure of Se4N4 is like that of S4N4 (15.59) with Se_N bond lengths of 180pm and cross-cage Se……Se separations of 276pm (compare with rcov)Se( = 117 pm). The reactivity of Se4N4 has not been as fully explored as that of S4N4. Reaction 15.118 is an adaptation of the synthesis of Se4N4 and leads to the 1,5-isomer of Se2S2N4 (1.1). In the solid state structure, the S and Se atoms are disordered making it difficult to tell whether the crystalline sample is Se2S2N4 or a solid solution of S4N4 and Se4N4. Mass spectrometric data are consistent with the presence of Se2S2N4, and the appearance of only one signal in the 14N NMR spectrum confirms the 1,5- rather than 1,3-isomer.
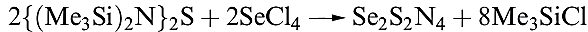 (1.1)
(1.1)
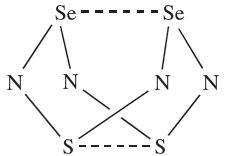
(1.1)



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|