


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 15-2-2018
Date: 6-7-2017
Date: 27-4-2019
|
Oxygen fluorides
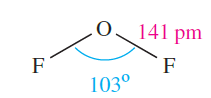
Oxygen difluoride, OF2 (1.1), is highly toxic and may be prepared by reaction 1.1. Selected properties are given in Table 1.1. Although OF2 is formally the anhydride of hypofluorous acid, HOF, only reaction 1.2 occurs with water and this is very slow at 298 K. With concentrated alkali, decomposition is much faster, and with steam, it is explosive.
(1.1)
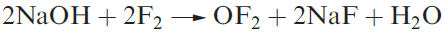 (1.1)
(1.1)
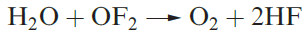 (1.2)
(1.2)
Pure OF2 can be heated to 470Kwithout decomposition, but it reacts with many elements (to form fluorides and oxides) at, or slightly above, room temperature. When subjected to UV radiation in an argon matrix at 4 K, the OF- radical is formed (equation 1.3) and on warming, the radicals combine to give dioxygen difluoride, O2F2.
 (1.3)
(1.3)
Dioxygen difluoride may also be made by the action of a high-voltage discharge on a mixture of O2 and F2 at 77–90K and 1–3 kPa pressure. Selected properties of O2F2 arelisted in Table 1.1.
Table 1.1 Selected physical properties of oxygen and sulfur fluorides.

The low-temperature decomposition of O2F2 initially yields O2F- radicals. Even at low temperatures, O2F2 is an extremely powerful fluorinating agent, e.g. it inflames with S at 93 K, and reacts with BF3 (equation 15.8) and SbF5 (reaction 1.4).
 (1.4)
(1.4)
The molecular shape of O2F2 (1.2) resembles that of H2O2 (Figure 15.9) although the internal dihedral angle is smaller (878). The very long O_F bond probably accounts for the ease of dissociation into O2F* and F*. Structures 1.3 show valence bond representations which reflect the long O_F and short O_O bonds; compare the O_O bond distance with those for O2 and derived ions (Section 15.4) and H2O2 (Table 15.3).
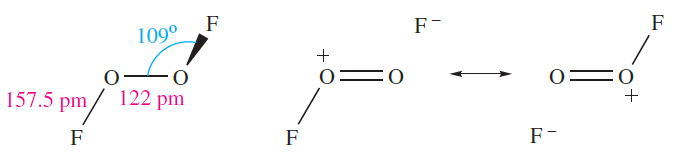
(1.2) (1.3)



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|