


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-12-2018
Date: 14-10-2018
Date: 4-3-2017
|
Extraction of the group 17 elements
Most fluorine-containing compounds are made using HF, the latter being prepared from fluorite by reaction 1.1; in 2001, ≈ 80% of CaF2 consumed in the US was converted into HF. Hydrogen fluoride is also recycled from Al manufacturing processes and from petroleum alkylation processes, and re-enters the supply chain. Difluorine is strongly oxidizing and must be prepared industrially by electrolytic oxidation of F- ion. The electrolyte is a mixture of anhydrous molten KF and HF, and the electrolysis cell contains a steel or copper cathode, ungraphitized carbon anode, and a Monel metal (Cu/Ni) diaphragm which is perforated below the surface of the electrolyte, but not above it, thus preventing the H2 and F2 products from recombining. As electrolysis proceeds, the HF content of the melt is renewed by adding dry gas from cylinders.
 (1.1)
(1.1)
We have already described the Downs process for extracting Na from NaCl and this is also the method of manufacturing Cl2 one of the most important industrial chemicals in the US. The manufacture of Br2 involves oxidation of Br- by Cl2, with air being swept through the system to remove Br2. Similarly, I- in brines is oxidized to I2. The extraction of I2 from NaIO3 involves controlled reduction by SO2; complete reduction yields NaI.
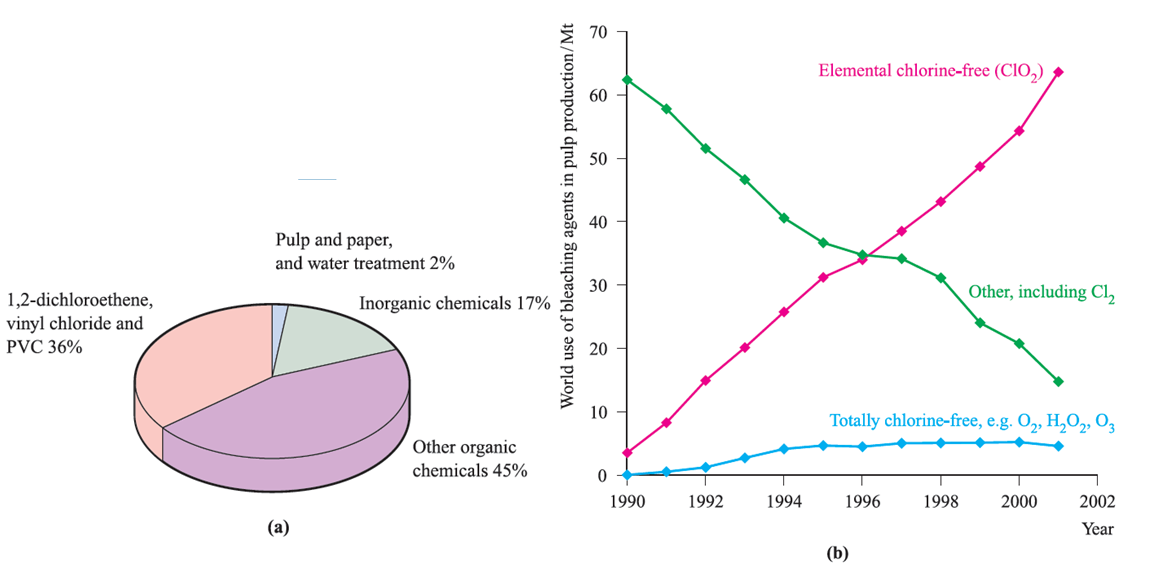
Fig. 1.1 (a) Industrial uses of Cl2 in Western Europe in 1994 [data: Chemistry & Industry (1995) p. 832]. (b) The trends in uses of bleaching agents in the pulp industry between 1990 and 2001; ClO2 has replaced Cl2. Both elemental chlorine-free and totally chlorine-free agents comply with environmental legislations [data: Alliance for Environmental Technology, 2001 International Survey].



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|