


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-12-2018
Date: 25-1-2019
Date: 28-12-2018
|
Oxofluorides
Oxofluorides are known for Xe(IV), Xe(VI) and Xe(VIII): XeOF2, XeOF4 , XeO2F2, XeO2F4 and XeO3F2. Their structures are consistent with VSEPR theory. The 1:1 reaction of XeF4 and H2O in liquid HF yields XeOF2, isolated as a pale yellow solid which decomposes explosively at 273K. In contrast to reaction 17.5, partial hydrolysis of XeF6 (equation 1.1) gives XeOF4 (a colourless liquid, mp 227 K), which can be converted to XeO2F2 by reaction 1.2. Reaction 1.3 is used to prepare XeO3F2 which can be separated in vacuo; further reaction between XeO3F2 and XeF6 yields XeO2F4.
 (1.1)
(1.1)
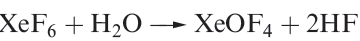 (1.2)
(1.2)
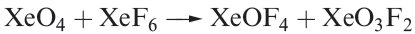 (1.3)
(1.3)
The stable salts M[XeO3F] (M = K or Cs) are obtained from MF and XeO3, and contain infinite chain anions with F- ions bridging XeO3 groups. Similar complexes are obtained from CsCl or RbCl with XeO3 but these contain linked [XeO3Cl2]2- anions as shown in 1.1.
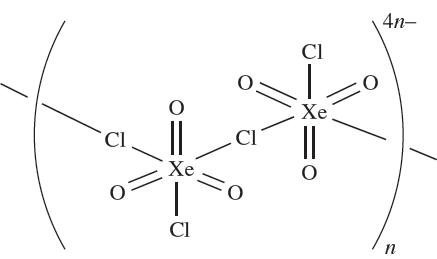
(1.1)



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|