


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-12-2016
Date: 4-9-2020
Date: 28-1-2019
|
Dealing with a Nuclear Breakup: Balancing Reactions
For purposes of this book, I define radioactivity as the spontaneous decay of an unstable nucleus. An unstable nucleus may break apart into two or more other particles with the release of some energy. This breaking apart can occur in a number of ways, depending on the particular atom that’s decaying.
If you know what one of the particles of a radioactive decay will be, you can often predict the other particle. Doing so involves something called balancing the nuclear reaction. (A nuclear reaction is any reaction involving a change in nuclear structure.) Balancing a nuclear reaction is really a fairly simple process. But before I explain it, here’s how to represent a reaction:
Reactants → products
Reactants are the substances you start with, and products are the new substances being formed. The arrow, called a reaction arrow, indicates that a reaction has taken place. For a nuclear reaction to be balanced, the sum of all the atomic numbers on the left-hand side of the reaction arrow must equal the sum of all the atomic numbers on the righthand side of the arrow. The same is true for the sums of the mass numbers.
Here’s an example: Suppose you’re a scientist performing a nuclear reaction by bombarding a particular isotope of chlorine (Cl-35) with a neutron. You observe that an isotope of hydrogen, H-1, is created along with another isotope, and you want to figure out what the other isotope is. The equation for this example is
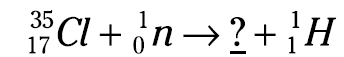
To figure out the unknown isotope (represented by ?), you need to balance the equation. The sum of the atomic numbers on the left is 17 (17 + 0), so you want the sum of the atomic numbers on the right to equal 17, too. Right now, you have an atomic number of 1 on the right; 17–1 is 16, so that’s the atomic number of the unknown isotope. This atomic number identifies the element as sulfur (S).
Now look at the mass numbers in the equation. The sum of the mass numbers on the left is 36 (35 + 1), and you want the sum of the mass numbers on the right to equal 36, too. Right now, you have a mass number of 1 on the right; 36 – 1 is 35, so that’s the mass number of the unknown isotope. Now you know that the unknown isotope is a sulfur isotope (S-35). And here’s what the balanced nuclear equation looks like:
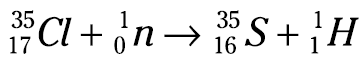
This equation represents a nuclear transmutation, the conversion of one element into another. Nuclear transmutation is a process human beings control. S-35 is an isotope of sulfur that doesn’t exist in nature. It’s a human-made isotope. Alchemists, those ancient predecessors of chemists, dreamed of converting one element into another (usually lead into gold), but they were never able to master the process. Chemists are now able, sometimes, to convert one element into another.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة إلى كلية مزايا الجامعة للمشاركة في حفل التخرج المركزي الخامس
|
|
|