
Energy Fluctuation in Canonical Ensemble
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 44
الجزء والصفحة:
part 2 , p 44
 26-8-2016
26-8-2016
 1780
1780
Energy Fluctuation in Canonical Ensemble
Show that for a canonical ensemble the fluctuation of energy in a system of constant volume is related to the specific heat and, hence, deduce that the specific heat at constant volume is nonnegative.
SOLUTION
First solution: For a canonical ensemble:
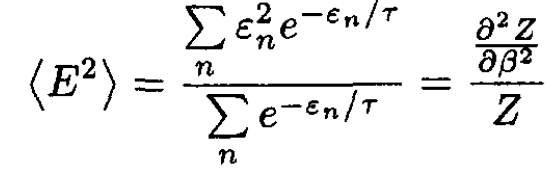 (1)
(1)
where β = 1/τ. On the other hand,
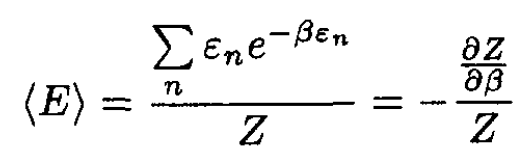 (2)
(2)
Differentiating (2), we obtain
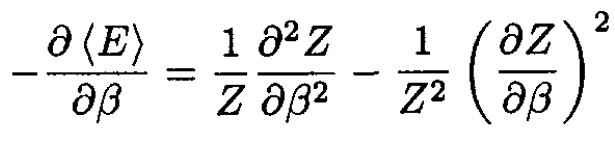 (3)
(3)
By inspecting (1)–( 3), we find that
 (4)
(4)
Now, the heat capacity at constant volume, Cv, is given by
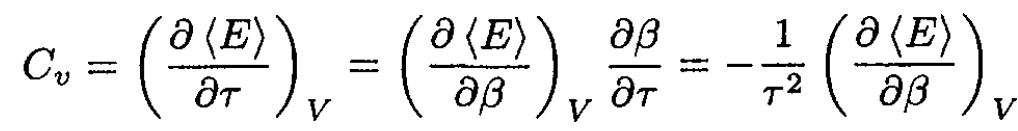 (5)
(5)
Therefore, comparing (4) and (5), we deduce that, at constant volume,
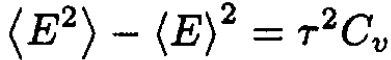 (6)
(6)
or in standard units
 (7)
(7)
Since
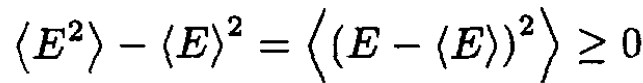
then
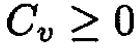
Second solution: A more general approach may be followed which is applicable to other problems. Because the probability ω of finding that the value of a certain quantity X deviates from its average value ⟨X⟩ is proportional to eS(X - ⟨X⟩) and denoting x = X - ⟨X⟩, we can write
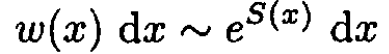 (8)
(8)
Note that ⟨X⟩ = 0. The entropy has a maximum at x = 0. Expanding S(x), we obtain
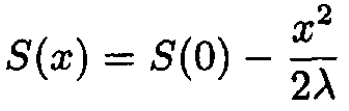
where

so λ > 0. The probability distribution
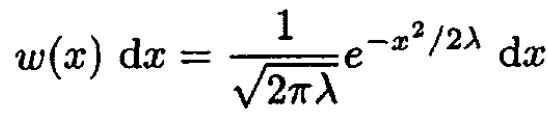 (9)
(9)
⟨x2⟩ = λ, so
 (10)
(10)
If we have several variables,
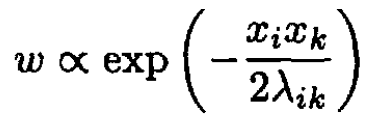 (11)
(11)
If the fluctuations of two variables xi, xk are statistically independent,

The converse is also true: If ⟨xixk⟩ = 0, the variables xi and xk are statistically independent. Now for a closed system we can write
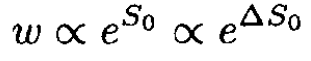 (12)
(12)
where S0 is the total entropy of the system and ∆S0 is the entropy change due to the fluctuation. On the other hand,
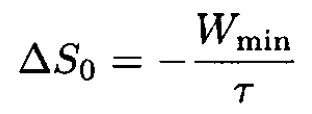 (13)
(13)
where Wmin is the minimum work to change reversibly the thermodynamic variables of a small part of a system (the rest of the system works as a heat bath), and τ is the average temperature of the system (and therefore the temperature of the heat bath). Hence,
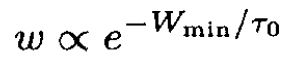
However,
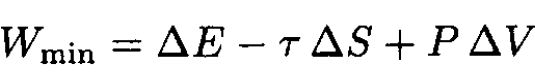 (14)
(14)
where ∆E, ∆S, and ∆V are changes of a small part of a system due to fluctuations and τ, P are the average temperature and pressure. So,
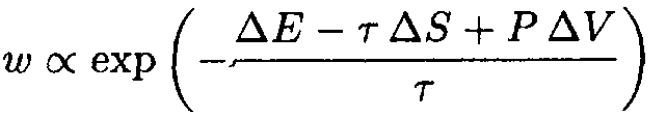 (15)
(15)
Expanding ∆E (for small fluctuations) gives
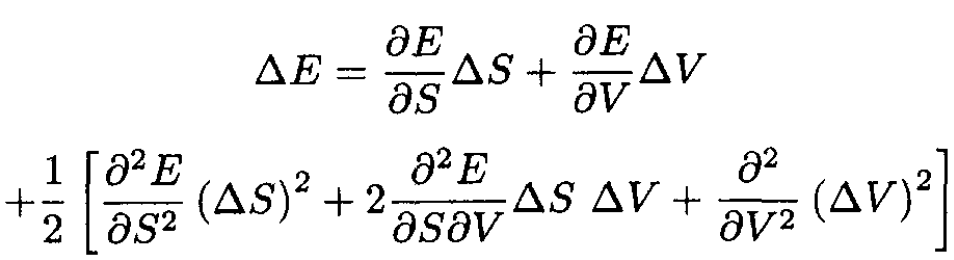 (16)
(16)
Substituting (16) into (14), we obtain
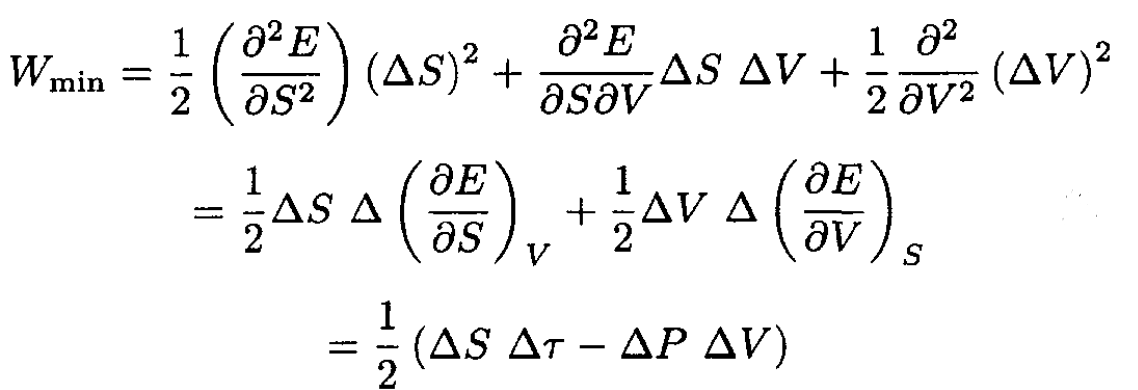 (17)
(17)
where we used

So, finally
 (18)
(18)
Using V and τ as independent variables we have
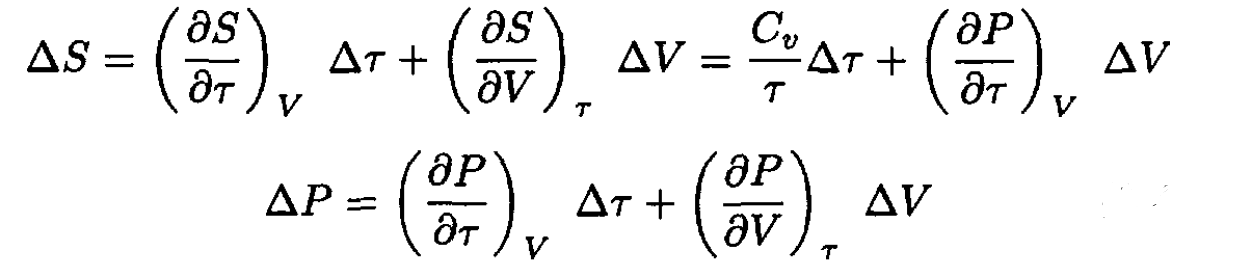 (19)
(19)
Substituting (19) into (18), we see that the cross terms with ∆V ∆τ cancel (which means that the fluctuations of volume and temperature are statistically independent, ⟨∆V. ∆τ ⟩ = 0):
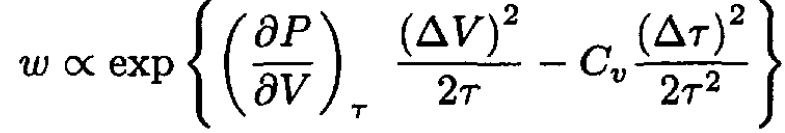 (20)
(20)
Comparing (20) with (10), we find that the fluctuations of volume and temperature are given by
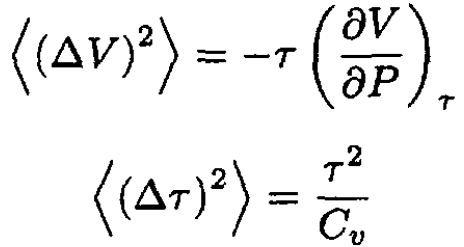 (21)
(21)
To find the energy fluctuation, we can expand ∆E:
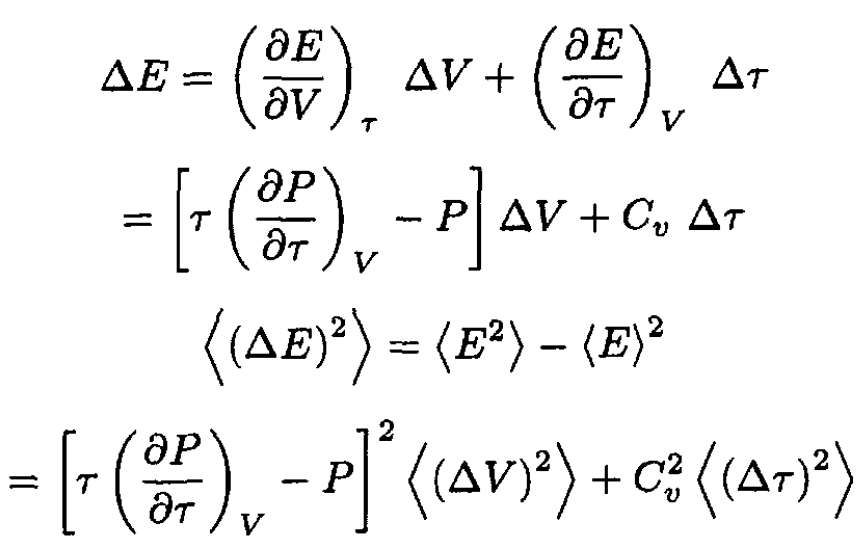 (22)
(22)
where we used ⟨∆V. ∆τ ⟩ = 0. Substituting 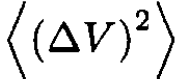 and
and  from (21), we obtain a more general formula for
from (21), we obtain a more general formula for 
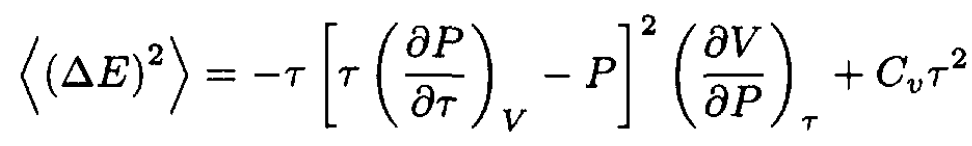 (23)
(23)
At constant volume (23) becomes
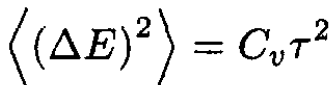
the same as before.
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


