


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 18-5-2016
Date: 24-10-2020
Date: 18-11-2020
|
The Particulate Nature of Gases, liquids and Solids
Everyday matter exists in three basic forms solids, liquids and gases and it is fairly easy to conjecture the essential particulate structure of these three forms.
Solids. The fundamental difference between a solid and a liquid or a gas is that a solid is, generally speaking, a rigid structure. The implication is that each of the component molecules is held roughly in the same place-the equilibrium position by the intermolecular force. The molecules are very close together (typically 10-10m) and are arranged in ordered ways (see figure 1.1(a)) extending over large numbers of particles. There are many patterns (or lattices) in which this can happen and this is reflected in the many examples of crystalline structure. Sometimes, however, in what are called amorphous solids, this order extends only over a few molecules, and the solid consists of myriads of very tiny crystals-crystallites-arranged in a random way. Also, difficulty in forming crystals occurs for some molecules. For example, in solids known as polymers the component molecules
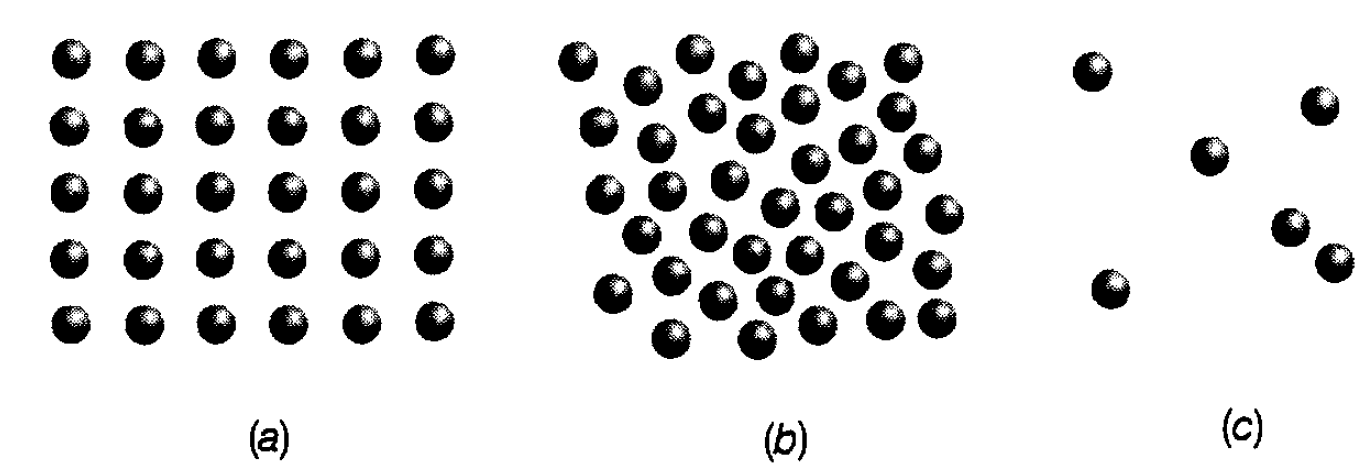
Figure 1.1: Instantaneous views of molecules in (a) a solid, (b) a liquid and (c) a gas.
are extremely complicated and irregularly shaped and are referred to as macromolecules. Packing them together in a tidy crystalline array is just not feasible. Finally, it must not be thought that the component molecules in a solid are at rest. Each molecule is always vibrating about its equilibrium position. It moves backwards and forwards between the sides of the valley of the relevant potential energy curve but does not escape from it.
Liquids. In a liquid the molecules are nearly as close to one another as in a solid but they frequently break away from their equilibrium positions. Generally speaking, each molecule is bonded to a few neighbouring molecules. However, the motion is much more complicated than in a solid and, as well as vibrating in relation to each other, the particles are also moving around in a very disordered way; an instantaneous impression of the way in which molecules are distributed is shown in figure 1.1(b). The nature of this motion is reflected in the lack of rigidity of a liquid. Its effect was dramatically demonstrated by an English botanist, Brown, in the early 19th century, who studied, microscopically, the dancing movement (Brownian motion) of grains of pollen suspended in water due to their collisions with the water molecules. It is, of course, difficult for the component molecules of a liquid to move about freely because of the drag of the attractive force and because of the very frequent intermolecular collisions-rather like people trying to move around in a crowd. This difficulty manifests itself as the phenomenon called viscosity which is the name for ‘resistance to flow’ exhibited to the extreme in substances such as treacle.
Gases. These have very low density (density is the mass per unit volume) compared with liquids and solids-they can even be ‘lighter than air’ (e.g. think of helium in a helium balloon). This means that the molecules are few and far between (see figure 1.1(c)); they are well separated (typically 3×10-9 m) compared with a solid or a liquid and, for most of the time, are too far apart to experience the intermolecular attractive force. The molecules are moving around in a random way all the time (typically with speeds around l02 m s-l) but collide with each other from time to time. The distance they travel on average between collisions is known as the mean free path; in the air that surrounds us this is a few hundred times the size of a molecule. A gas can easily be compressed compared with a liquid or solid and, given the structure just envisaged, this is to be expected; there is plenty of empty space within the gas in which to bring the molecules together. It must also be recognized that the continual pounding of the moving molecules on the surface of any container exerts an outward force on it; this is simply what we know as the pressure due to the gas. The magnitude of the pressure is defined as the force exerted per square metre of the surface. Why at ordinary room temperature some things are gases, others liquids and others solids relates simply to the strength of the intermolecular attractive force. There are two competing features to consider: the motion of the individual particles and the attraction of the intermolecular force. The motion tends to keep the molecules separated from each other whilst the force tends to draw them together. In gases the motion wins (the force is weak); in solids the force wins (the force is strong) and in liquids we have what might be called the ‘hallway house’ when both activities are equally important.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|