


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2-5-2016
Date: 27-11-2020
Date: 16-5-2016
|
FK506
The immunosuppressive drug FK506 (also known as tacrolimus) is a 23-membered macrocyclic lactam (Fig. 1) that was first isolated from a strain of Streptomyces tsukubaensis in Japan in 1987 (1). In addition to blocking the T cell immune response, FK506 has neuroprotective properties (2, 3( and antiparasitic effects (4). It was first used clinically for immunosuppression in 1989. The target of FK506 for immunosuppression is thought to be the cytosolic FK506-binding protein, FKBP12, which is also a peptidyl prolyl cis/ trans isomerase (PPIase). Upon binding to FKBP12 within a deep hydrophobic pocket, the imide bond of FK506 adopts a trans conformation, concomitant with reorganizing the conformation of the pipecolinic ring and of the pyranose ring. Recently, a cell-permeable, covalently linked dimer of FK506 (FK1012) was constructed as a tool to achieve controlled cross-linking of intracellular receptor domains for gene activation (5). It utilizes the high binding affinity of FK506 for the receptor FKBP12, which is effective at less than nanomolar concentrations. A number of derivatives that bind to and inhibit FKBP have been reported, some immunosuppressive and others not (6) .
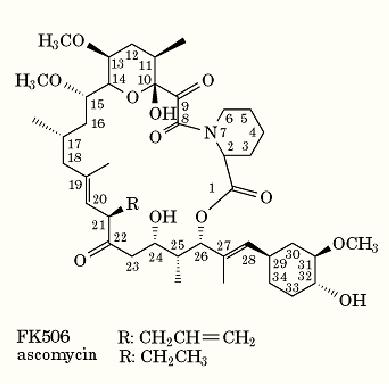
Figure 1. The structure of immunosuppressant FK506
Despite structural similarities to rapamycin in a major part of the macrocycle, many biological effects of FK506, including the arrest of clonal T cell expansion, are reminiscent of another class of immunosuppressants, cyclosporin A (CsA), rather than rapamycin. This finding was the first evidence of a common upstream target for the two different classes of immunosuppressants, FK506 and cyclosporin A. As monitored by affinity chromatography, the FKBP12/FK506 complex binds the same protein from a calf thymus extract, the protein phosphatase calcineurin, as has been obtained with the complex of CsA and its target cyclophilin Cyp18. In both cases noncompetitive inhibition by the complex is observed in the nanomolar concentration range. Considering that the structures of Cyp18/CsA and FKBP12/FK506 are unrelated in amino acid sequence and spatial organization, the similar inhibition of calcineurin is striking. FK506 acts as a pro-drug, in that only the complex of the drug and its receptor is active. Two segments of FKBP12 comprising the loops in the region of residues 40 and 80 plus an effector region of FK506, the carbon chain C17 to C32, may be involved in drug activation. In the X-ray crystallographic structure of the human FKBP12/FK506/calcineurin complex, the FKBP12/FK506 portion contacts two distinct areas on calcineurin that do not directly obstruct both the active site and the substrate-binding cleft of the protein phosphatase. On the contrary, blocking a rather distant subsite of the phosphatase/protein phosphate recognition region is responsible for phosphatase inhibition (8).
Both CsA and FK506 block the induction of clonal T cell expansion at the early G0 to G1 transition of the cell cycle. They exert down-regulation of transcription of a number of similar lymphokine genes.
References
1. T. Kino et al. (1987) J. Antibiot. 40, 1249–
2. W.E. Lyons et al. (1994) Proc. Natl. Acad. Sci. USA 91, 3191–3195.
3. J. Sharkey and S.P. Butcher (1994) Nature 371, 336–339.
4. A. Moro et al. (1995) EMBO J. 14, 2483–2490.
5. D.M. Spencer, T.J. Wandless, S.L. Schreiber, and G.R. Crabtree (1993) Science 262, 1019–1024.
6. D.M. Armistead and M.W. Harding (1993) Ann. Rep. Med. Chem. 28, 207–215.
7. J. Liu et al. (1991) Cell 66, 807–817.
8. C.R. Kissinger et al. (1995) Nature 378, 641–644.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|