


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 7-12-2015
Date: 1-3-2021
Date: 16-12-2015
|
Colicins
Colicins produced by and active against coliform bacteria constitute a subset of the bacteriocins generated by many groups of bacteria. They have been a subject of interest since very early in this century. In 1925, Gratia demonstrated that Escherichia coli strain V (virulent in experimental infection) in liquid media produces a heat-stable substance that in high dilution inhibits the growth of E. coli (1). This protein was designated as colicin V. It has now been shown that it fits better with the description of the so-called “microcins” (2). Then a whole series of colicins produced by E. coli and closely related members of Enterobacteriacae were discovered. Mainly as a result of the influence and efforts of Fredericq (3), knowledge of the colicins advanced at a great rate, and more than 20 different types were identified on the basis of their action against a set of specific resistant (generally receptor-deficient) mutants (Table 1). In contrast to the bacteriocins from Gram-positive bacteria that kill species other than those that are likely to have the same ecological niche, colicins are active only against E. coli and closely related bacteria (there is a similar relationship between the cloacins and Enterobacter cloacae, the klebicins and Klebsiella species, and the pyocins and pseudomonacae). A characteristic feature of colicinogenic bacteria is to be specifically immune to the colicin they make but not to other colicins. A large number of colicins have now been identified, and each has been characterized by the corresponding specific immunity protein. Colicins are plasmid-encoded, and each plasmid also bears an immunity protein, thus ensuring that plasmid carriers are protected from the colicin they themselves produce. There is probably a selective advantage for Enterobacteriacae to produce a colicin because 30 to 40% of natural isolates of E. coli carry colicinogenic plasmids (4). The colicins exert their lethal effect through a single-hit mechanism (5). There was some controversy about the meaning of this terminology, but now there is a consensus that sensitive cells are killed by a single event, implying that a single molecule kills, although not every one does.
Table 1. Characteristics of Colicins
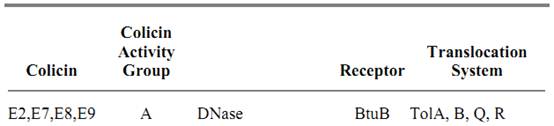
Colicins are soluble proteins of 29 to 70 kDa (for colicin V). Their amino acid sequence is known, and they share the property of being linearly organized in three domains that have specific functions. They are also highly asymmetrical molecules and have axial ratios of 8 to 10 (6, 7) . Another common feature is that their production is induced by exposure of colicinogenic bacteria to agents like UV light and genotoxic chemicals, such as mitomycin C, that elicit the SOS response. After induction, they are produced in large amounts, so they provide useful model systems to study fundamental biological problems, such as protein–protein interactions, polypeptide translocation and insertion across and into membranes, functioning of voltage-gated pores, etc.
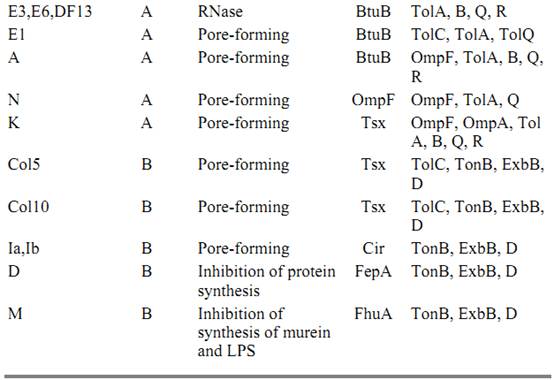
The classification of colicins reflects bacterial rather than colicin properties. According to its activity spectrum against a variety of mutants, a particular colicin is unambiguously assigned to one of two groups, A and B (8, 9). Group B colicins are inactive on strains that have a lesion in the tonB gene but are active against strains mutant in tolA and tolB genes. Group A colicins show the opposite specificity. Now we know that group A colicins (A, E1 to E9, K, L, N, and cloacin DF13) and filamentous bacteriophage (f1, fd, and M13) need the Tol proteins to penetrate into cells (9-11). Group B colicins (B, D, Ia, Ib, M, 5, and 10) and phages T1 and F80 need TonB and its associated proteins (8, 9). In all cases studied so far, the determinants for colicinogeny are located on plasmids (Table 1) of two different types: small multicopy plasmids or large low-copy-number plasmids that generally correspond to the A and B groups of colicins. In general, group A colicins are encoded by small plasmids and are actively released to the extracellular medium, whereas group B colicins are encoded by large plasmids and are very poorly secreted.
At least three hypotheses have been proposed to explain the evolution of colicin plasmids: (1( positive selection of diversity, (2) recombinational shuffling, and (3) transposition (12). The different colicins may have evolved by DNA recombination of fragments encoding different colicin domains. This is best exemplified by the common uptake route for colicins B and D, which have a highly homologous N-terminal and central polypeptide sequence (defining the translocation and receptor domains respectively) but very different C-terminal domains with different types of activities (13).
1. Genes for Colicins and Associated Proteins
Regardless of the plasmid type, the genes for the colicin, immunity, and lysis (when there is one) are always clustered (14). The difference between immunity to the enzymatic colicins and to the channel-forming colicins and colicin M (whose target is the cytoplasmic membrane) is reflected in the regulation of their synthesis and the arrangement of the various colicin operons. In enzymatic colicins, the immunity gene is transcribed in the same direction as the colicin and lysis genes (15-17), whereas for the channel-forming colicins and for colicin M, immunity is encoded on the opposite DNA strand and is thus transcribed in the direction opposite to the colicin and lysis genes (18-20) . Enzymatic colicin and cognate immunity genes form an operon under the control of an SOS promoter. Thus, induction of colicin synthesis results in increasing the amount of immunity protein synthesized, but the presence of a terminator causes partial transcription arrest upstream of the lysis gene. In contrast, inducing the synthesis of pore-forming colicins from their SOS promoter does not result in a concomitant increase in the immunity protein. The immunity is constitutively expressed from a weak promoter (19, 21-23). The situation is similar for the murein-synthesis-inhibiting colicin M and its immunity gene (24).
Colicin production is inducible and suicidal. Under normal conditions, very few cells produce colicin because the expression is repressed by the LexA repressor and is switched on only during the SOS response normally associated with repairing damaged DNA. This means that, in culture, colicin production is inducible in a growing population of cells by mutagens (UV light, mitomycin C). This induction is very efficient. In the absence of mutagens, LexA exhibits a very tight repression (25( and ensures that only a small proportion of cells go down the dead-end road of colicin expression and export. Once they have done so, it is important to the plasmid clone as a whole that the sacrificial cells produce as much colicin as possible. This maximizes selection against non-plasmid-bearing cells. Meanwhile, the silent preservation of many identical copies of the same plasmid in immune cells ensures no significant loss of the plasmids in the producer cell.
2. Extracellular Release of Colicins
Most of the colicins (group A, but not group B) are actively released into the growth medium. Their release mechanism differs from that of proteins secreted by Gram-negative bacteria:
1. they do not contain an N-terminal or C-terminal signal sequence like Sec-dependent exported polypeptides or those depending on ABC transporters;
2.their release depends on the function of a lytic protein (variously called kil, lys, brp) whose gene is part of the colicin operon, downstream of the colicin gene;
3. they are released from the host cell some time after synthesis;
4. their extracellular release is not specific, and quasi-lysis proteins have various effect on cells in addition to causing the release of assorted proteins and small molecules (26).
The lysis genes encode short proteins produced in a precursor form with a signal sequence and a typical cysteine lipid-modification consensus box, implying that the precursor undergoes the following posttranslational modifications: (1) acylation of the cysteine residue, (2) cleavage of the signal sequence by signal peptidase II, and (3) fatty acylation of the amino group of the now N-terminal acylated cysteine. The maturation and processing of lysis proteins occurs slowly, so every intermediate form can be observed. Both the mature form and the signal sequence accumulate in E. coli after processing (27, 28). The addition of globomycin to E. coli cells stops processing of lysis protein precursors and inhibits colicin release (29). Acylation is essential for quasi-lysis (29, 30). The mature lysis proteins activate the normally dormant phospholipase A in the outer membrane of colicin-producing cells, thus causing production of lysophospholipids, quasi-lysis, and colicin release (31). However, lysis proteins must also affect the permeability of the inner membrane (32). The lysis protein must reach a critical concentration within the cells before quasi-lysis and colicin release, which also indicates that the lysis protein directly affects the inner membrane. Local modifications in the structure of the bilayer are induced by interactions with lipopeptide micelles, as previously suggested in the case of Iturin A and bacillomycin L (33).
3. Mechanism of Entry and Domain Organization of Colicins
Colicins bind to specific receptors in the bacterial outer membrane, from where they are translocated to, and eventually through, the inner membrane to reach their targets. Consistent with these three steps, their polypeptide chains are linearly organized into three domains: the N-terminal domain is involved in the translocation step, the central domain is responsible for receptor binding, and the C-terminal domain carries the lethal activity (Fig. 1). The boundaries of these domains were defined first by limited proteolytic digestion (34-36), then by genetic engineering (37, 38), and from sequence homologies between colicins with the same type of lethal activity and using the same receptors (E-type, for example) or the same translocation pathways. Even colicin M, which has a molecular mass of 29 kDa, has three functional domains (24).
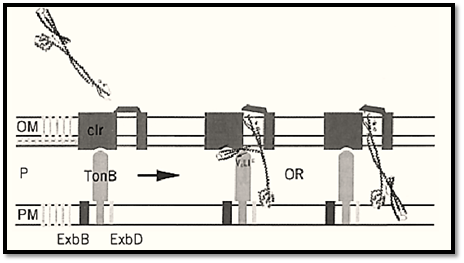
Figure 1. The mechanism of colicin Ia attachment and translocation. The outer membrane (OM) receptor for colicin Ia consists of Cir, TonB, and accessory plasma membrane (PM) proteins ExbB and ExbD. The translocation domain (blue), receptor binding domain (green), and channel-forming domain (red) separated by a pair of helices (gray), each 16 nm long, are indicated in colicin Ia. The TonB box of colicin Ia may compete with the TonB box of Cir for linkage to TonB. The channel-forming C domain reaches the plasma membrane, where it forms an ion-conducting channel by subsequent insertion and rearrangement of helices within the membrane (not shown). The translocation T domain may remain near the periplasmic surface of the outer membrane during channel activity. However, the presence of the 16 nm-long T3 helix indicates an alternative possibility in which the T domain crosses the periplasmic space (P) to participate in channel formation in vivo. The location of the TonB box on the upper surface of the T domain of sequence Glu(E)-Ile(I)-Met(M) Ala(A)-Val(V) is indicated, reading right to left, as EIMAV. The arrows indicate the locations of the TonB box in the receptor and colicin Ia. Reproduced from (68) with permission.
To enter the cells, colicins have borrowed multiprotein systems used by sensitive cells for important biological functions. These proteins include porins, vitamin B12, siderophore, nucleoside receptors, and multiprotein systems that cooperate with these proteins. The group A and group B colicins correspond to two different pathways of entry beyond receptor binding: the TonB and the Tol pathway. The receptors for the various colicins are given in Table 1. The protein most frequently used is BtuB, the vitamin B12 receptor, which defines the so-called E-type colicins (E2 to E9) (12). High-affinity receptors or iron siderophores are also used by many colicins. The nucleoside Tsx porin and the major porin OmpF are also receptors (Table 1). Depending on the colicin class, the colicin receptors function cooperatively with either the Ton B system or the Tol system.
The tol locus was originally defined as comprising four loci, tol A, B, Q, R, on the basis of group A colicin tolerance (39). These genes are organized in two operons that comprise three additional genes called orf1, orf2 and pal (40), whose gene products are not directly involved in colicin uptake. However, one of them, the Pal protein (peptidoglycan associated protein), a lipoprotein of the outer membrane, interacts with TolB (41). Both tol and pal mutants have an altered outer membrane (39). The only soluble Tol protein is TolB, which is principally located in the periplasm (42) but is linked to the outer membrane through interaction with Pal. The other Tol proteins are associated with the cytoplasmic membrane. TolQ contains three membrane-spanning segments, and TolA and TolR are anchored to this membrane by their N-terminal regions. All three proteins interact through membrane-spanning a-helices (43, 44). There is yet no direct evidence that TolB interacts with membrane-associated Tol proteins, but each of the Tol proteins cofractionates with a membrane fraction accounting for the presumed contact sites between the inner and outer membranes of E. coli. In colicin A-treated cells, the toxin was also found in this fraction, and the relative amount of Tol proteins was doubled, suggesting that the colicin itself may recruit more Tol proteins at the contact sites (45, 46).
Although both TolB and TolR are required to take up most group A colicins, neither is required for colicin E1, which in contrast requires the so-called TolC protein, a minor outer membrane protein that forms pores in vitro (47) and is also involved in secreting hemolysin (48) and colicin V (49). OmpF, another porin, is required for translocating many group A colicins (A, E-type, N) (50, 51). The N-terminal domain of group A colicins contains all the information needed for the translocation step (52) beyond receptor binding, including binding to OmpF (or TolC for colicin E1) and to Tol proteins like TolA (53) and TolB (54). There are about 1000 translocation sites for colicin A per bacterium (55). In addition, it has been shown that: (1) unfolding occurs upon receptor binding (55), (2) the N-terminal domain interacts with Tol proteins, (3) the polypeptide chain spans the entire cell envelope after inserting the pore-forming domain (56), and (4) a reduced constriction of the lumen of the OmpF pore prevents translocation of colicin A and N (57). A hypothetical model of colicin translocation based on these results has been presented (11, 58.(
Group B colicins are imported into sensitive cells through high-affinity siderophore receptors and the Ton B pathway. The Ton B system consists of a complex of proteins TonB-ExbB-ExbD, which facilitates the flow of energy from the cytoplasmic to the outer membrane for the energy-dependent transport of ferric siderophores and of vitamin B12. ExbB and ExbD are physically and functionally homologous to TolQ and TolB (59). Ton B, like Tol A, has its N-terminal anchor in the cytoplasmic membrane and spans the periplasm (60). It has been proposed that an energized conformation of TonB, like FepA or FhuA, opens channels in the outer membrane, which act as receptors for the ferric siderophores. Until recently, each of the group B colicins studied uses a Ton B-dependent receptor, but it has now been reported that a new colicin (colicin 10) uses the Ton B system and binds to a Ton B-independent receptor Tsx. Colicin 10 also requires TolC for its uptake (61). Thus the interaction between the colicin receptor and Ton B is not obligatory if TolC is involved. This situation may be comparable to that of the group A colicin E1, which does not need TolB or TolR but does need TolC.
The high-affinity receptors for siderophores and vitamin B12 contain a pentapeptide motif close to the N-terminus, called the “Ton B box” (62). Mutations in the Ton B box reduce receptor activity dramatically. They are also suppressed by mutations in TonB (63), indicating physical and
functional interactions between TonB and the receptors. Group B colicins, such as colicins B and D, which bind to the FepA receptor, and colicin M, which binds to the FhuA receptor, also contain a TonB box sequence close to the N-terminus. This indicates that these colicins interact directly with TonB during translocation. Mutations in the TonB box of colicin B affect its uptake and are suppressed by secondary substitutions clustered in TonB (63). A similar situation is observed with TonB, FhuA, and colicin M. These results suggest that group B colicins need to interact with TonB to be translocated, just as group A colicins need to interact with Tol proteins. Both the N- and C-terminal domains of the protein also interact with the inner membrane and therefore are protected from proteolytic degradation (64). This suggests that the Y-shaped structure (65) allows colicin Ia to span the entire cell envelope, as previously reported with colicin A (66). The receptor-binding domain would be the only part remaining exposed outside the cells.
A molecular model of colicin M uptake has been proposed (24). After first binding to FhuA in the outer membrane, colicin M is taken up in an energy-coupled process through the action of the TonB protein, which is anchored to the inner membrane and spans the periplasmic space. TonB binds to FhuA, inducing the release conformation of FhuA, which, in turn, results in the vectorial translocation of colicin M across the outer membrane. TonB itself has two conformations, energized and unenergized. Energization of TonB takes place in the cytoplasmic membrane. Induction of the receptor release conformation consumes energy, so TonB dissociates from the FhuA receptor and has to be reenergized to induce the next round of colicin M uptake. Because both FhuA and colicin M contain a TonB box, the TonB protein most likely interacts with both of them sequentially. The role of ExbB and D is still unclear. They are involved either in energy transduction (cycling of the TonB conformational change) from the cytoplasmic membrane to TonB or in stabilizing TonB, which is physically and functionally unstable (24, 67, 68).
The 626-residue colicin Ia is about 21 nm long and consists of three functional domains separated by a pair of a-helices 16 nm long. A central domain at the bend of the hairpinlike structure mediates binding to the outer membrane receptor. A second domain mediates translocation across the outer membrane via the TonB pathway. The TonB box recognition motif of colicin Ia is on one side of three 8 nm-long helices arranged as a helical sheet. The third domain, made up of 10 a-helices, is the pore-forming domain. The two exceptionally long 16 nm a-helices enable colicin Ia to span the periplasmic space between the outer and inner membranes (Fig. 1) (68). This type of structure, which will very likely be found in each colicin, strongly suggests how these toxins exploit the machinery of the target cell to get across the periplasmic space (Fig. 1).
4. Mode of Action of Colicins
The biochemical effects of colicins on sensitive bacteria fall into four classes: (1) the RNase colicins typified by colicin E3, (2) DNase colicins typified by colicins E2 and E9, (3) pore-forming colicins like colicin E1, and the still unique colicin M, which inhibits cell wall biosynthesis (24).
4.1RNase Colicins
The two most extensively studied members of this class are colicin E3 and cloacin DF13 (derived from a strain of Enterobacter cloacae closely related to E. coli). The 11 kDa and 12 kDa C-terminal segments of these bacteriocins cleave the 16S ribosomal RNA, either in isolation or in intact 30S ribosomal subunits, which explains their inhibitory effect on protein synthesis (69). Colicins E5 and E6 have the same type of activity (70). Although they are highly homologous in their RNase regions (71), colicins E3, E5, E6, and cloacin DF13, each have a specific immunity protein. No structures are yet available for any of these RNase domains.
4.2. DNase Colicins
In 1970, it was shown that colicin E2 causes both single- and double-strand breaks in the DNA of sensitive cells (72), and three new types of DNase colicins were subsequently described (colicin E7, E8, and E9). The sequential identity between the various DNase domains is greater than 80% (71), yet each is inhibited by a specific immunity protein. This is similar to the situation in the RNase-type colicins. Structural and biophysical analysis of the DNase immunity protein complexes has been aided by the E. coli overexpression systems developed by Wallis et al. (73-75).
4.3. Pore-Forming Colicins
Pore-forming colicins constitute the largest group. Colicin V must be set apart in this class because it fits the definition of microcins better than that of colicins (2). Pore-forming colicins dissipate the membrane potential (76-80), which causes a series of metabolic effects, such as inhibition of active transport and of protein and nucleic acid synthesis, decrease of the internal ATP concentration, and leakage of potassium ions (81-85). Cell death results from the depletion of the ATP pool following efflux of phosphate through the channel and ATP hydrolysis (86). Ten pore-forming colicins have been identified thus far. Sequential homologies indicate that they should be separated into two groups: (1) E1, 5, Ia, Ib and (2) A, B, and N (87), differing mainly in a segment containing the loop between helices 8 and 9, as defined in the X-ray crystal structure of the C-domain of colicin A (88). The structures of soluble forms of the channel domains of colicins A, Ia and E1, are known to varying extents (89-92). The pore-forming domain is composed of a bundle of 10 a-helices arranged in three layers in a novel protein fold (93). The N-terminal layer (helices 1 and 2)is connected to another layer consisting of two pairs of amphipathic helices (helices 3 + 4 and ،(6 + 7 and a single helix (helix 5) connects the two helix pairs. The middle layer is composed of helices 8 and 9, which form a hydrophobic hairpin buried in the core of the molecule (Fig. 1). The external sides of the peripheral helices are hydrophilic, which explains the paradox of how the same protein domain exists either in a water-soluble form or in a membrane-inserted state. Because the C-terminal domain of colicins is easily purified by proteolytic digestion of the whole colicins or by genetic engineering, many biophysical studies have been carried out to improve our understanding of this step in the action of pore-forming colicins.
Schein et al. (78) were the first to demonstrate that colicin A forms well-defined channels in planar lipid bilayers that permit ions to cross the membrane (94, 95). Pore-forming colicins form channels of 10 to 30 picosiemens (pS), which correspond to 107 ions/sec/channel. These channels are characterized by their sensitivity to the electrical potential across the membrane. The gating voltage ) corresponding to the activation or to the inactivation of 50% of the channels) varies for a given colicin. Values are +21, +50, and +70 mV for colicins N, A, and E1, respectively. There are multiple membranes states at the single-channel level. With colicin E1, channels of 10 pS are initially observed. With increased activation in 1 M KCl, channels of 20, 40, and 60 pS with substates appear, and flickering is observed. Similar observations with other colicins suggested the existence of many closed, open, and inactivated states. Thus, the concept of a single-membrane-inserted conformation appears to be incorrect, although a simplified model with a single or a few membrane-inserted forms has been very helpful.
The channel properties of the C-domain and of whole colicin are qualitatively similar for colicin E1, but not for colicins A (96) and Ia (97). Differences are ascribed to the influence of the other domains on the pore-forming domain in the intact molecule. The pores are permeable to cations and anions, but the rates at which they are transported are low (98). There is a relative preference for cations versus anions that is modulated by the pH and the lipid composition (99, 100). Using the relative permeabilities of large organic cations and anions and taking into account their asymmetrical shapes, the size of the channel diameter was estimated at only 0.4–0.5 nm. This implies strong interactions between permeable ions and the side chains of residues exposed in the channel lumen (100.(
The transition of pore-forming colicins from a water-soluble to a membrane-inserted state involves large structural changes, which is of interest in the context of protein folding, protein translocation, and protein insertion into membranes. From studies with model membrane systems, a likely sequence of events for membrane insertion and pore formation has been proposed for colicins A and E1. Briefly, the C-domain first binds to the outer face of the cytoplasmic membrane. The negative charge density plays a part in the binding and the kinetics of insertion (101, 102). After binding, but before insertion, the pore-forming domain must have lost the tertiary structure of the water-soluble form and adopted primarily a molten globular conformation, which is still compact and has kept its native secondary structure (87, 103, 104). As mentioned later, the interaction of the N-terminal and central domains with the translocation machinery might trigger the appearance of a molten globular conformation of the channel domain in vivo (105). The topology of the membrane-inserted state has been extensively studied, mainly with the pore-forming domains of colicins E1, A, and Ia. The membrane-bound state involves insertion of the hydrophobic helical hairpin formed by H8 and H9 into the bilayer. In contrast to colicin A, which is more dependent on electrostatic interactions, the protein would lie flat on the membrane surface. The hydrophobic hairpin could not insert because the density of charged residues on the membrane surface is too high (103) .
The polypeptide translocation involved in voltage-dependent gating has been studied through three main experimental approaches: (1) By using lipophilic radiolabeled probes, Merill and Cramer (106) demonstrated that a 42-residue region of colicin E1 corresponding to helices 5 and 6 was labeled in the presence, but not in the absence, of a membrane potential in a vesicle system with valinomycin-induced diffusion potential. Another segment corresponding to the hydrophobic hairpin was strongly labeled in the presence or absence of membrane potential. (2) The existence of a drastic change triggered by a transnegative membrane potential in vivo was also demonstrated by disulfide-bond engineering (107). The same technique further showed that a-helices 1, 2, 3, and 10 remain at the membrane surface after application of a membrane potential. (3) Colicin Ia with biotin, conjugated to specific cysteine residues introduced by site-directed mutagenesis, were inserted into a lipid bilayer, and the channels were opened or closed by varying the membrane potential. Then streptavidin was added on the cis- or trans-side of the membrane. The results demonstrated (108) that a region of the pore-forming domain of colicin Ia reversibly flips across the membrane, implying a large conformational change when potential is applied. A region of at least 68 residues is able to flip back and forth with channel opening and closing. Several open, channel structures probably exist (109).
4.4. Inhibition of Murein Biosynthesis
Colicin M is unique among the colicins in that it inhibits murein biosynthesis (110) by interfering with the dephosphorylation of C55-polyisoprenyl pyrophosphate, which leads to cell lysis (111). It also has a much shorter polypeptide chain than other colicins (29 kDa as compared to 40 to 70 kDa). Yet, it has the same basic design of three functional domains (see previous). The largest part of the C-terminal domain of colicin M resides in the cytoplasmic membrane because the carrier lipid and pyrophosphatase activity were found in the membrane fraction (112). Colicin M does not penetrate deeply into the cytoplasmic membrane but acts mostly at the periplasmic side of this membrane (24). Furthermore, cytoplasmic colicin M does not kill the cells, and only external colicin M, after its import through the energy-dependent TonB system, has access to the target in the cytoplasmic membrane. A chimeric protein of colicin M linked to a signal peptide directing the C-domain to the periplasm (the outer face of inner membrane) causes cell lysis. The amount of colicin M at the target was so high that immunity broke down (24).
5. The Systems Immune to Colicins
The immunity of colicinogenic cells to the action of the colicin they produce was first noted by Fredericq in 1957 (113). The cell relies for its survival on the immunity protein. The individual cell is safe from its own colicin when that gene is carried on the same plasmid as the imm gene. Much progress with regard to the specificity determinants has been made during recent years on the basis of the immune activity, especially for nuclease-type and pore-forming colicins.
5.1. Immunity Against RNases
The determinants for specificity of colicin-immunity interactions with colicins E3, E6 and cloacin DF13 are likely to reside in a limited number of amino acid residues, eight in the nuclease domains and up to nine in the corresponding immunity proteins (114, 115). These determinants are located in the N-terminal regions of both the RNase domain and the immunity protein. A single residue either in Im3 or in Im6 is critical for defining the specificity (116). Other residues are also likely to be involved in the interaction but have more peripheral roles in defining specificity. The 84-residue Im3 (117) is folded into a four-stranded antiparallel b-sheet connected by loops and a single short a-helix, in marked contrast to the structure of a DNase-specific immunity protein Im9 (118), which contains four a-helices. The specificity-determining residues of Im3 at the two main positions are exposed and line one face of the b-sheet (117). No structures are yet available for any RNase domains.
5.2. Immunity Against DNases
The sequences of the DNase domains of colicins E2, E7, E8, and E9 are more than 80% identical, yet they have specific immunity proteins. Site-directed mutagenetic studies identified six Col E9 residues in the C-terminal DNase domain as possible specificity determinants (119). The sequences of Im2, Im7, Im8, and Im9 are 58% identical but are not homologous to the RNase-type immunity proteins. Two amino acid residues were identified as specificity determinants of Im8 and Im9 by using homologous recombination (74) and site-directed mutagenesis (118, 120).
Further structural and biophysical studies of DNase-immunity protein complexes have shown that the DNase E9-Im9 complex is extremely stable (like the Col E9-Im9 complex) with a Kd of× 9.3 10-17 M, one of the highest affinities ever measured for a protein interaction (121). The association of the nuclease with the Im9 protein is essentially diffusion-controlled, involves electrostatic steering, and follows a two-step mechanism in which the proteins form an initial encounter complex before undergoing a conformational change to yield the final stable complex (121) . Although there is no cross-reactivity between DNase-type colicins (E2, E7, E8, and E9) and noncognate immunity proteins under normal levels of expression, biophysical studies indicated that the latter bind to the DNase domain of colicin E9 and inhibit its activity (122). The Kd values range from 10–17 M (IM9) to 10–4 M. Consistent with this result, overexpressing each of the noncognate Im genes in bacteria results in significant levels of cross-reactivity toward the ColE9 toxin, and the order of in vivo cross-reactivity (Im9 > Im2 > Im8 > Im7) mirrors exactly the measured in vivo affinities (122). The specificity for the DNase-Im complexes is controlled through the dissociation constant, which is more than 106-fold faster for the noncognate Im proteins. The Im9 fold consists of a distorted antiparallel four-helix bundle in which the second helix (from the N-terminus) is the main specificity determinant (118, 120). The surface regions of Im9 that interact with the DNase include the two central helices of the molecule, and the binding surface is heavily negatively charged, consistent with a positively charged partner DNase domain (118).
5.3. Immunity to Pore-Forming Colicins
The immunity proteins directed against nuclease-type colicins described previously are expressed at a level similar to that of their cognate colicins. In contrast, the proteins immune to pore-forming colicins are expressed constitutively at very low levels (102 to 10 3 molecules per cell). Another major difference is that these proteins protect the cells against external colicin because the membrane potential has the wrong orientation for colicin activity from the inside. Thus the immunity is directed against colicins produced by other cells.
Membrane vesicles from cells immune to colicin Ia are depolarized by colicin E1, but not Ia (123), which first indicated a cytoplasmic membrane localization for these types of immunity proteins. In addition, the construction of hybrids between colicins Ia and Ib, A and E1, or A and B, demonstrated that, independent of the translocation pathway, immunity is directed specifically against the C-terminal, pore-forming domain (19, 52, 124). ImmA has four transmembrane a-helices, and both the N- and C-termini are located in the cytoplasm (125). The shorter ImE1 has three transmembrane a-helices. The N- and C-termini are on the cytoplasmic and periplasmic sides of the membrane, respectively (127). These orientations are in agreement with the inside-positive prediction rule (128).
As with Im proteins directed against nucleases, there is no cross-reactivity between homologous immunity proteins directed against homologous A, B, or N colicins. Similarly, overproduction of the immunity protein leads to partial cross-reactivity (124). The main determinant for specific immunity recognition is located in the hydrophobic hairpin of the channel domain of the colicin (124, 129). Either the whole bacteriocin or the C-terminal domain of pore-forming colicins produced in the cytoplasm of E. coli are devoid of cytotoxicity. However, when the C-terminal domain is fused to a signal sequence, the channel is inserted and functional, and cell death follows. This could be inhibited by coproduction of the cognate immunity protein (130, 131). The cytotoxocity of the hybrid protein is independent of the uptake machinery normally used, demonstrating that the C-domain alone forms the channel in vivo, as in in vitro. The interaction of ImA with colicin A requires the immunity protein to assemble functionally but does not require the channel to be in the open state (131) . In other words, the immunity protein interacts with the hydrophobic helical hairpin, as first suggested by the studies mentioned previously (124). This interaction was demonstrated directly using an epitope-tagged immunity protein (131). Site-directed mutagenetic studies of ImA indicate that a role for polar regions of ImA cannot be excluded, in addition to the intramembrane helix–helix interactions. Two roles are proposed for the hydrophilic loops in this protein: (1) they may stabilize its interactions with the channel on both sides of the membrane; (2) they may be required for the functional assembly of the transmembrane helices of ImA (132). The colicin E1 immunity protein tolerates a higher degree of substitution than ImA (127).
5.4. Immunity to Colicin M
Like the protein it inhibits, the immunity to colicin M is unique when compared with other Im proteins. It prevents colicin M from inhibiting murein synthesis. This 14 kDa protein has been localized in the cytoplasmic membrane, and a substantial portion is exposed to the periplasmic space. There is indirect evidence that the colicin M-immunity interaction occurs at the periplasmic side of the cytoplasmic membrane (133).
References
1. A. Gratia (1925) C.R. Soc. Biol. 93, 1041–1041.
2. R. Kolter and F. Moreno (1992) Ann. Rev. Microbiol. 46, 141–163.
3. P. Fredericq (1963) Ann. Rev. Microbiol. 11, 7–22.
4. M. Riley and D. Gordon (1992) J. Gen. Microbiol. 138, 1345–1352.
5. F. Jacob, L. Siminovitch, and L. Wollman (1952) Ann. Inst. Pasteur 83, 295–315.
6. J. Konisky (1982) Ann. Rev. Microbiol. 36, 125–144.
7. D. Cavard, P. Sauve, F. Heitz, F. Pattus, C. Martinez, R. Dijkman, and C. Lazdunski (1988(Eur. J. Biochem. 172, 507–512.
8. J. K. Davis and P. Reves (1975) J. Bacteriol. 123, 96–101.
9. J. K. Davis and P. Reeves (1975) J. Bacteriol. 123, 102–117.
10. R. Nagel del Zwaig and S. E. Luria (1967) J. Bacteriol. 94, 1112–1123.
11. C. Lazdunski (1995) Mol. Microbiol. 16, 1059–1066.
12. M. A. Riley (1993) Mol. Biol. Evol. 10, 1048–1059.
13. U. Ross, E. E. Harkness, and V. Braun (1989) Mol. Microbiol. 3, 891–902.
14. S. Luria and J. Suit (1987) In Escherichia coli and Salmonella typhimurium (F. E. Neidhardt, ed.), ASM, Washington, DC, pp. 1615–1624.
15. H. Masaki and T. Ohta (1985) J. Mol. Biol. 82, 217–227.
16. S. T. Cole, B. Saint-Joanis, and A. P. Pugsley (1985) Mol. Gen. Genetics 198, 465–472.
17. K. F. Chak and R. James (1985) Nucleic Acids Res. 13, 2519–2530.
18. P. T. Chan, H. Ohmori, J. I. Tomizawa, and J. Lebowitz (1985) J. Biol. Chem. 260, 8925–8935.
19. J. A. Mankovich, C. H. Hsu, and J. Konisky (1986) J. Bacteriol. 168, 228–236.
20. J. Morlon, M. Chartier, M. Bidaud, and C. Lazdunski (1988) Mol. Gen. Genetics 211, 231–243.
21. R. Lloubes, D. Baty, and C. Lazdunski (1986) Nucleic Acids Res. 14, 2621–2636.
22. A. P. Pugsley (1988) Mol. Gen. Genetics 211, 335–341.
23. S. Zhang, L. Yan, and G. Zubay (1988) J. Bacteriol. 170, 5460–5467.
24. V. Braun, S. Gaisser, C. Glaser, R. Harkness, T. Ölschager, and J. Mende (1992) In Bacteriocins, Microcins and Lantibiotics (R. James, C. Lazdunski, and F. Pattus, eds.), Springer-Verlag, Berlin, Heidelberg, NATO ASI Series, Vol. 65, pp. 119–125.
25. R. Lloubes, M. Granger-Schnarr, C. Lazdunski, and M. Schnarr (1991) J. Mol. Biol. 217, 421– 428.
26. D. Cavard and B. Oudega (1992) In Bacteriocins, Microcins and Lantibiotics (R. James, C. Lazdunski, and F. Pattus, eds.), Springer-Verlag, Berlin, Heidelberg, NATO ASI Series, Vol. 65, pp. 297–305.
27. B. Oudega, A. Ykema, F. Stegehuis, and F. de Graaf (1984) FEMS Microbiol. Lett. 22, 101–108.
28. D. Cavard, R. Lloubes, J. Morlon, M. Chartier, and C. Lazdunski (1985S. P. Howard, D. Cavard, and C. Lazdunski ) Mol. Gen. Genet. 199, 95–100.
29. D. Cavard, D. Baty, S. P. Howard, H. Verheij, and C. Lazdunski (1987) J. Bacteriol. 169, 2187–2194.
30. A. P. Pugsley and S. T. Cole (1987) J. Gen. Microbiol. 133, 2411–2420.
31. A. P. Pugsley and M. Schwartz (1984) EMBO J. 3, 2393–2397.
32. (1991) J. Gen. Microbiol. 137, 81–89.
33. R. Maget-Dana, F. Heitz, M. Ptak, F. Peypoux, and M. Guinaud (1985) Biochem. Biophys. Res. Commun. 129, 965–971.
34. Y. Ohno-Iwashita and K. Imahori (1980) Biochemistry 19, 652–659.
35. F. de Graaf and B. Oudega (1986) Curr. Top. Microbiol. Immunol. 125, 183–205.
36. R. Dreher, V. Braun, and B. Wittman-Liebold (1985) Arch. Microbiol. 140, 343–346.
37. D. Baty, M. Frenette, R. Lloubes, V. Géli, S. P. Howard, F. Pattus, and C. Lazdunski (1988( Mol. Microbiol. 2, 807–811.
38M. Frenette, H. Bénédetti, A. Bernadac, D. Baty, and C. Lazdunski (1991) J. Mol. Biol. 217, 2509–2514 .
39. R. Webster (1991) Mol. Microbiol. 5, 1005–1011.
40. A. Vianney, M. Michelle Muller, T. Clavel, J. C. Lazzaroni, R. Portalier, and R. E. Webster (1996) J. Bacteriol. 178, 4031–4038.
41. E. Bouveret, R. Derouiche, A. Rigal, R. Lloubes, C. Lazdunski, and H. Bénédetti (1995) J. Biol. Chem. 270, 11071–11077.
42. M. Isnard, A. Rigal, J. C. Lazzaroni, C. Lazdunski, and R. Lloubes (1994) J. Bacteriol. 176 6392–6396 .
43. R. Derouiche, H. Bénédetti, J. C. Lazzaroni, C. Lazdunski, and R. Lloubes (1995) J. Biol. Chem. 270, 11078–11084.
44. J. C. Lazzaroni, A. Vianney, J. L. Popot, H. Bénédetti, S. Samatey, C. Lazdunski, R. Portalier, and V. Géli (1995) J. Mol. Biol. 246, 1–7.
45. J. P. Bourdineaud, S. P. Howard, and C. Lazdunski (1989) J. Bacteriol. 171, 2458–2465.
46. G. Guiard, P. Boulanger, H. Bénédetti, R. Lloubes, M. Besnard, and L. Letellier (1993) J. Biol. Chem. 269, 5874–5880.
47. R. Benz, E. Maier, and I. Gentscher (1993) Zbl Bakt 278, 187–196.
48. C. Wandersman and P. Deleplaire (1990) Proc. Natl. Acad Sci. USA 87, 4776–4780.
49. L. Gilson, H. Mahanty, and R. Kolter (1990) EMBO J. 9, 3875–3884.
50. H. Bénédetti, M. Frenette, D. Baty, R. Lloubes, V. Géli, and C. Lazdunski (1989) J. Gen. Microbiol. 135, 3413–3420.
51. J. P. Bourdineaud, H. P. Fierobe, C. Lazdunski, and J. M. Pagès (1990) Mol. Microbiol. 4, 1737-1743.
52. H. Bénédetti, M. Frénette, D. Baty, R. Lloubes, M. Knibiehler, F. Pattus, and C. Lazdunski (1991) J. Mol. Biol. 217, 429–439.
53. H. Bénédetti, C. Lazdunski, and R. Lloubes (1991) EMBO J. 10, 1989–1995.
54. E. Bouveret, A. Rigal, C. Lazdunski, and H. Bénédetti (1997) Mol. Microbiol., 23, 909–920.
55. D. Duché, D. Baty, M. Chartier, and L. Letellier (1994) J. Biol. Chem. 269, 24820–24825.
56. H. Bénédetti, R. Lloubes, C. Lazdunski, and L. Letellier (1992) EMBO J. 11, 441–447.
57. D. Jeanteur, T. Schirmer, D. Fourel, V. Simonet, G. Rummel, C. Widner, J. P. Rosenbusch, F. Pattus, and J. M. Pagès (1994) Proc. Natl. Acad. Sci. USA 91, 10675–10679.
58. H. Bénédetti, L. Letellier, R. Lloubes, V. Géli, D. Baty, J. M. Pagès, and C. Lazdunski (1992(In Dynamics of Membrane Assembly (J.A.F. Op den Kamp, ed.), NATO ASI Series, Springer-Verlag, Berlin, Vol. 63, pp. 316–332.
59. K. Eick-Helmerich and V. Braun (1995) J. Bacteriol. 171, 5117–5126.
60. K. Postle (1990) Mol. Microbiol. 4, 2019–2925.
61. H. Pils and V. Braun (1995) Mol. Microbiol. 16, 57–67.
62. E. Schramm, J. Mende, V. Braun, and R. M. Kemp (1987) J. Bacteriol. 169, 3350–3357.
63.V. Braun (1995) FEMS Microbiol. Rev. 16, 295–307.
64. S. F. Mel, A. M. Falick, A. L. Burlingame, and R. M. Stroud (1993) Biochemistry 312, 9473–9479 .
65. P. Ghosh, S. Mel, and R. M. Stroud (1994) Nature Struct. Biol. 1, 597–604.
66. H. Bénédetti, R. Lloubes, C. Lazdunski, and L. Letellier (1992) EMBO J. 11, 441–447.
67. K. Postle and J. Skare (1988) J. Biol. Chem. 263, 11000–11007.
68. M. Wiener, D. Freymann, P. Ghosh, and R. Stroud (1997) Nature 385, 461–464.
69. K. Jakes (1982) In Molecular Action of Toxins and Viruses (P. Cohen and S. von Heinegen, eds.), Elsevier, Amsterdam.
70. M. Mock and A. P. Pugsley (1982) J. Bacteriol. 150, 1069–1076.
71. P. C. K. Lau, M. Parsons, and T. Uchimura (1992) In Bacteriocins, Microcins and Lantibiotics (R. James, C. Lazdunski, and F. Pattus, eds.), Springer-Verlag, Berlin, pp. 353–378.
72. P. S. Ringrose (1970) Biochim. Biophys. Acta 213, 320–334.
73.R. Wallis, A. Reilly, A. Rowe, G. Moore, R. James, and C. Kleanthous (1922) Eur. J. Biochem. 207, 687–695.
74. R. Wallis, G. R. Moore, C. Kleanthous, and R. James (1992) Eur. J. Biochem. 210, 925–930.
75. R. Wallis, A. Reilly, K. Barnes, C. Abell, D. Campbell, G. Moore, R. James, and C. Kleanthous (1994) Eur. J. Biochem. 220, 447–454.
76. J. Weiss and S. Luria (1978) Proc. Natl. Acad. Sci. USA 75, 2483–2487.
77. H. Tokuda and J. Koninsky (1978) Proc. Natl. Acad. Sci. USA 76, 6167–6171.
78. S. Schein, B. Kagan, and A. Finkelstein (1978) Nature 276, 159–163.
79. W. Cramer, J. Dankert, and Y. Uratami (1983) Biochim. Biophys. Acta 737, 173–193.
80. J. P. Bourdineaud, P. Boulanger, C. Lazdunski, and L. Letellier (1990) Proc. Natl. Acad. Sci. USA 87, 1037–1041.
81. K. Fields and S. Luria (1969) J. Bacteriol. 97, 57–63.
82. K. Fields and S. Luria (1969) J. Bacteriol. 97, 64–77.
83. A. Kopecky, D. Copeland, and J. Lusk (1975) Proc. Natl. Acad. Sci. USA 72, 4631–4634.
84. C. Plate, J. Suit, A. Jetten, and S. Luria (1974) J. Biol. Chem. 19, 6138–6143.
85. J. Gould and W. Cramer (1977) J. Biol. Chem. 252, 5491–5497.
86. G. Guihard, H. Bénédetti, M. Besnard, and L. Letellier (1994) J. Biol. Chem. 268, 17775–17780.
87. C. Lazdunski, D. Baty, V. Géli, D. Cavard, J. Morlon, R. Lloubes, P. Howard, M. Knibiehler, M. Chartier, S. Varenne, M. Frenette, J. L. Dasseux, and F. Pattus (1988) Biochim. Biophys. Acta 947, 445–464.
88. M. Parker, F. Pattus, A. Tucker, and D. Tsernoglou (1989) Nature 337, 93–96.
89. M. Parker, J. Postna, F. Pattus, A. Tucker, and D. Tsernoglou (1992) J. Mol. Biol. 224, 639–657.
90. P. Ghosh, S. Mel, and R. Stroud (1994) Nat. Struct. Biol. 1, 597–504.
91. P. Elkins, H. Y. Song, W. Cramer, and C. Stauffacher (1994) Proteins Struct. Funct. Genet. 19, 150-157.
92. M. Wormald, A. Merril, W. Cramer, and R. Williams (1990) Eur. J. Biochem. 191, 155–161.
93. L. Holm and C. Sander (1993) FEBS Lett. 315, 301–306.
94. F. Pattus, D. Cavard, R. Verger, C. Lazdunski, and H. Schindler (1983) in Physical Chemistry of Transmembrane Ion Motions (G. Spach, ed.), Elsevier, Amsterdam, pp. 407–413.
95. F. Pattus, D. Massote, H. Wilmsen, J. Lakey, D. Tsernoglou, A. Tucker, and M. Parker (1990(Experientia 96, 180–192.
96. M. Collarini, G. Amblard, C. Lazdunski, and F. Pattus (1987) Eur. Biophys. J. 14, 147–153.
97. P. Gosh, S. Mel, and R. Stroud (1993) J. Membrane Biol. 134, 85–92.
98. L. Raymond, S. Slatin, and A. Finkelstein (1985) J. Membrane Biol. 84, 173–181.
99. J. Bullock (1992) J. Membrane Biol. 125, 255–257.
100. J. Bullock, E. Kolen, and J. L. Shear (1992) J. Membrane Biol. 128, 1–16.
101. F. van der Goot, N. Didat, F. Pattus, W. Dowhan, and L. Letellier (1993) Eur. J. Biochem. 213, 217-221.
102. G. van der Goot, J. Gonzalez-Manas, J. Lakey, and F. Pattus (1991) Nature 354, 408–410.
103. J. Lakey, G. van der Goot, and F. Pattus (1994) Toxicology 87, 85–108.
104. M. Parker and F. Pattus (1993) Trends Biochem. Sci. 18, 391–395.
105. D. Duché, D. Baty, M. Chartier, and L. Letellier (1994) J. Biol. Chem. 269, 24820–24825.
106. A. Merill and W. Cramer (1990) Biochemistry 29, 8529–8534.
107. D. Duché, M. Parker, J. Gonzalez-Manas, F. Pattus, and D. Baty (1994) J. Biol. Chem 269, 6332-6339.
108. S. Slatin, X-Q. Qiu, K. Jakes, and A. Finkelstein (1994) Nature 371, 158–161.
109. X. Q. Qiu, K. Jakes, P. Kienker, A. Finkelstein, and S. Slatin (1996) J. Gen. Physiol. 107, 313–328.
110. K. Schaller, J. Höltje, and V. Braun (1982) J. Bacteriol. 152, 994–1000.
111. R. Harkness and V. Braun (1989) J. Biol. Chem. 264, 6177–6182.
112. G. Siewert and J. Strominger (1967) Proc. Natl. Acad. Sci. USA 57, 767–773.
113. P. Fredericq (1957) Ann. Rev. Microbiol. 11, 7–21.
114. H. Masaki, S. Yajima, A. Akutsur-Koide, T. Ohta, and T. Uozumi (1992) In “Bacteriocins, Microcins and Lantibiotics” (R. James, C. Lazdunski, and F. Patus eds.), Springer-Verlag, Berlin, pp. 379–395.
115. A. Akutso, H. Masaki, and T. Ohta (1989) J. Bacteriol. 171, 6430–6436.
116. H. Masaki, A. Akutsu, T. Uozumi and T. Ohta (1991) Gene 107, 133–138.
117. S. Yajima, Y. Muto, S. Yokoyama, H. Masaki, and T. Uozumi (1992) Biochemistry 31, 5578–5586.
118. M. Osborne, A. L. Breez, L. Y. Lian, A. Reilly, R. James, C. Kleanthous, and G. Moore (1996(Biochemistry 35, 9505–9512.
119. M. Curtis and R. James (1991) Mol. Microbiol. 5, 2727–2733.
120. M. Osborne, L-Y. Lian, R. Wallis, A. Reilly, R. James, C. Kleanthous, and G. Moore (1994(Biochemistry 33, 12347–12355.
121. R. Wallis, G. Moore, R. James, and C. Kleanthous (1995) Biochemistry 34, 13743–13750.
122. R. Wallis, K. Y. Leung, A. Pomoner, H. Videler, G. Moore, R. James, and C. Kleanthous (1995) Biochemistry 34, 13751–13759.
123. C. Weaver, A. Redborg and J. J. Konisky (1981) J. Bacteriol. 148, 817–828.
124. V. Géli and C. Lazdunski (1992) J. Bacteriol. 174, 6432–6437
125. V. Géli, D. Baty, and C. Lazdunski (1988) Proc. Natl. Acad. Sci. USA 85, 689–693.
126.V. Géli, D. Baty, F. Pattus, and C. Lazdunski (1989) Mol. Microbiol. 3, 679–687.
127. H. Song and W. Cramer (1991) J. Bacteriol. 173, 2935–2943.
128. G. von Heijne (1992) J. Mol. Biol. 225, 487–494.
129. Y. Zhang and W. Cramer (1993) J. Biol. Chem. 268, 1–8.
130. D. Espesset, Y. Corda, K. Cunningham, H. Bénédetti, R. Lloubes, C. Lazdunski, and V. Géli (1994) Mol. Microbiol. 13, 1121–1131.
131. D. Espesset, D. Duché, D. Baty, and V. Géli (1996) EMBO J. 15, 2356–2364.
132. D. Espesset, P. Piet, C. Lazdunski, and V. Géli (1994) Mol. Microbiol. 10, 1111–1120.
133. T. Ölschläger, A. Turba, and V. Braun (1991) Mol. Microbiol. 5, 1105–1111.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|