


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-6-2021
Date: 16-6-2021
Date: 9-11-2020
|
Bifunctional Crosslinking Reagents
1.Terminology
If a bifunctional chemical crosslinking agent is designed so that its two reactive groups are identical, it is referred to as a homobifunctional reagent; if its two reactive groups are different, it is a heterobifunctional reagent. If one or both reactive groups of the crosslinker become so only as the result of a photochemical reaction caused by exposing the reagent to light of an appropriate wavelength, then the crosslinking reagent is photoactivatable, photoreactive, photosensitive, or light-activated (caged). The portion of the crosslinking reagent other than, and usually between, its two reactive groups is referred to as the spacer arm, or crossbridge. If the spacer arm contains a covalent bond that can be easily broken (by an oxidant, reductant, base, etc), then the crosslinker is termed cleavable; otherwise, it is classified as noncleavable. With most chemical crosslinkers, a portion of the reagent is incorporated into the final crosslinked complex; however, there is a special class of reagents that facilitate crosslinking without being incorporated into the final covalent complex. These reagents are referred to as zero-length crosslinkers, because the groups they crosslink are not separated by a spacer arm.
2.Variables in the Design of Crosslinking Reagents
By varying the chemical and physical properties of the reactive groups and spacer arm of a crosslinking agent, one can obtain crosslinkers with different properties and uses. A heterobifunctional reagent, with its two different types of reactive groups, allows for different selectivities in the functional groups that become crosslinked, compared to a homobifunctional reagent containing a pair of the same reactive groups. The selectivity shown by the reactive groups of crosslinkers for the side chains of particular amino acids mirrors the selectivity of those reactive groups when used as monofunctional reagents in the chemical modification of proteins, from which crosslinking is an outgrowth.
A number of variables pertain to spacer arms: (1) the length of the crossbridge, as in the case of bisimidoesters (Table 1) with different numbers of methylene groups in the spacer arm; (2) its geometry, which in the case of the phenylenebismaleimides (Table 1) also causes an increase in length, progressing from ortho to para; (3) its chemical nature, especially the number of charged or polar groups on the spacer arm, which influences the crosslinker's solubility properties and uses. For example, membrane proteins would more likely be crosslinked by bifunctional reagents that are hydrophobic, rather than hydrophilic. Reporter groups (chromophores, radioactive tracers, etc) can also be incorporated into spacer arms to facilitate quantification of the extent of protein modification, studies on the crosslinked complex, or identification of the crosslinked components. Incorporation into the spacer arm of cleavable bonds, such as disulfide bonds, is also useful in the identification of crosslinked components, especially by diagonal methods (1).
Table 1. Examples of Bifunctional Crosslinkers
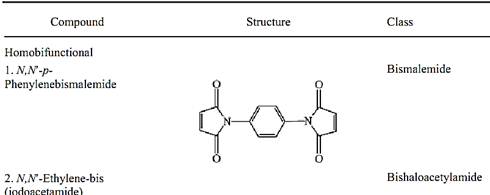

3.Examples of Bifunctional Crosslinkers
The compounds listed in Table 1 are representative of some of the most frequently used chemical classes of crosslinking reagents, but they represent only a small fraction of the literally hundreds of bifunctional crosslinking reagents that have been described in the scientific literature. The widely used N-substituted bismaleimides are selective for thiol groups, especially at pH values near neutrality, where amino groups are predominantly protonated. This class of crosslinkers is available with many different spacer arms, having large variations in length and solubility, or having chromophores or cleavable bonds incorporated. The alkylating bishaloacetyl crosslinkers are also selective at neutral pH for sulfhydryl groups, but they will also react with imidazole side chains of some histidine residues under this condition. It should be remembered that few reagents used to modify proteins chemically are absolutely specific for any given amino acid side chain; on the other hand, high selectivity can often be achieved empirically through varying the pH, reaction time, and concentrations of reactants.
The bisimidoesters were among the earliest used crosslinkers and are also available with a variety of spacer arms. These compounds preferentially react at alkaline pH with amino groups, with which they form amidine bonds, retaining the positive charge of the original amine. A more frequently used class of amine-selective reagents is the N-hydroxysuccinimide esters, which produce an amide-crosslinked product that is relatively stable in aqueous solution. The N-hydroxysuccinimide ester group is frequently combined with maleimide, such as in compound 6 of Table 1, to produce heterobifunctional crosslinkers with selectivity toward crosslinking amino and sulfhydryl groups. Another common class of heterobifunctional crosslinkers contains at one end a photoactivatable group, which is generally assumed to be nonselective for the functional groups it modifies. For this class of reagents, the two-step reaction protocol is particularly useful, with the chemical reaction being carried out first in the absence of activating radiation, followed by irradiation of the photosensitive group to bring about crosslinking. The arylazide shown in Table 1 (compound 7) is an example of a photosensitive crosslinker that is widely used. The bis-homobifunctional aldehyde glutaraldehyde (Table 1, compound 5) is perhaps the most frequently used chemical crosslinker.
References
1. J. A. Cover, J. M. Lambert, C. M. Norman, and R. R. Traut (1981) Biochemistry 20, 2843–2852.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|