


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2-11-2020
Date: 29-4-2021
Date: 21-3-2021
|
Alpha-Bungarotoxin and Curare-Mimetic Toxins
The venom of the banded krait snake (Bungarus multicinctus) contains a variety of protein toxins that are collectively called bungarotoxins. As is often the case, an animal venom is, in fact, a mixture of different toxins specific for different target molecules. The a-neurotoxins, or curare-mimetic toxins, bind specifically to the acetylcholine receptor with high affinities (Kd in the 10–9 to 10–12 M range for neuronal and muscular receptors of different species) and prevent the opening of the ion channel caused by acetylcholine binding (1, 2). Such inhibition of the transmission of the nerve-muscle impulse results in a flaccid paralysis, which may end in respiratory failure and death.
a-bungarotoxin is the prototype of a large group of monomeric toxins, produced by many snakes, including cobra and sea snakes, and composed of 66 to 74 amino acid residues with four or five disulfide bonds. Their three-dimensional structure is rather flat, with three adjacent b-sheet loops (3) that expose residues essential for receptor binding: Lys-27, Trp-29, Arg-39, and Lys-55 (numbering of erabutoxin) (Fig. 1) (4).
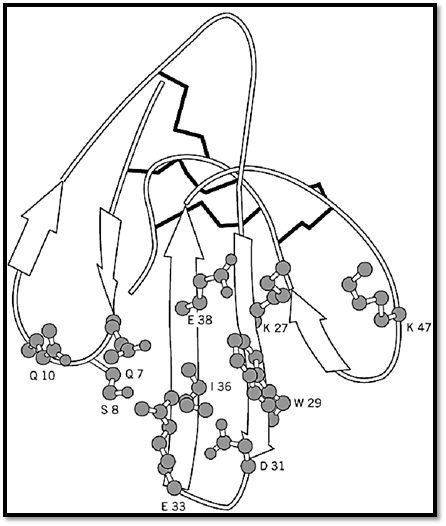
Figure 1. Erabutoxin, a snake toxin that binds to the acetylcholine receptor. Folding of the polypeptide chain shows the typical three-finger folding of curare-mimetic snake toxins. Several residues determine the specific binding and inhibition of the acetylcholine receptor with Lys-27, Trp-29, Arg-33, and Lys 47 playing a major role (4). Reproduced from (2) with permission.
Many species of snakes, including B. multicinctus, also produce a-neurotoxins of smaller size. Their polypeptide chains are 60 to 62 residues long but adopt the same three-finger b-sheet fold, stabilized by four disulfide bonds. At variance from the longer a-neurotoxins, these toxins are dimers, but the same three positively charged residues are essential for receptor binding. The corresponding area of the acetylcholine receptor involved in a-neurotoxin binding has not been mapped in detail, but the segment of residues 185 to 199 plays a major role, together with residues 128 to 142.
The various a-neurotoxins bind with a range of affinities and specificities to the multitude of muscular and neuronal acetylcholine receptors; fluorescent and radiolabeled toxin derivatives are invaluable tools for the study of the structure and function of such receptors (5).
References
1. A. L. Harvey, ed. (1991) Snake Toxins, Pergamon Press, New York.
2. R. Rappuoli and C. Montecucco (1997) Guidebook to Protein Toxins and Their Use in Cell Biology, Oxford University Press, Oxford, UK.
3. M. D. Walkinshaw, W. Saenger, and A. Maelicke (1980) Proc. Natl. Acad. Sci. USA 77, 24002404- .
4. O. Tremeau et al. (1995) J. Biol. Chem. 270, 9362–9369.
5. A. Devillers-Thiery et al. (1993) J. Membr. Biol. 136, 97–112.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|