


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 20-11-2015
Date: 18-11-2015
Date: 19-11-2015
|
Flaviviruses
Viruses in the flavivirus family (Flaviviridae) include the genera Flavivirus, Hepacivirus, and Pestivirus. Flaviviruses (the prototype being the yellow fever virus [Latin: flavus, yellow]) are transmitted by arthropods. They cause a bi- phasic infection that can have serious consequences (hemorrhagic fever with a high lethality rate). In southern and eastern countries, these viruses are significant human pathogens. Only one representative of this family, the tick- borne encephalitis pathogen, is encountered in Europe.
The hepaciviruses (hepatitis C [HCV] and hepatitis G viruses) are not arthropodborne. HCV is transmitted mainly in blood (transfusions, blood products, intravenous drug use) and is a frequent cause of chronic disease (70% of cases), including cirrhosis of the liver and hepatocellular carcinoma. The hepatitis G virus (HGV) is related to HCV and has not been characterized in detail as yet.
Pestiviruses are only important in veterinary medicine.
Flaviviruses show morphological uniformity with an icosahedral capsid and closefitting, spiked envelope. The size of the capsid is about 30 nm and the whole virion measures 45 nm. The genome of the flaviviruses is a singlestranded sense RNA about 10 kb in size. It codes for three structural and seven nonstructural proteins. Both cotranslational and posttranslational protein processing , similar to what is seen in the picornaviruses, has been described. The morphogenesis of the virus occurs at the endoplasmic reticulum, into the lumen of which the finished viruses bud. These characteristics have not been directly demonstrated for the hepatitis C virus, which cannot be cultured in vitro.
The pestiviruses cause severe animal epidemics (e.g., swine fever). They are not transmitted by arthropods.
Flavivirus (Arthropodborne Yellow Fever Type)
Pathogen. The flavivirus family includes 63 species, among them the prototypic virus of the family, the yellow fever virus, and the pathogen that causes European tickborne encephalitis (spring-summer meningoencephalitis, SSME). Table 2 lists the flaviviruses that cause significant travelers' diseases.
Pathogenesis and clinical picture. The arthropodborne flaviviruses cause diseases of different levels of severity. The infections are typically biphasic with an initial, not very characteristic phase including fever, headache, muscle pain, and in some cases exanthem (Denguelike disease). This phase includes a pronounced viremia. The illness, in this stage often not recognized as a flavivirus infection, may then be over or it may progress after one to three days to a second, severe clinical picture: a hemorrhagic fever with a high lethality rate involving hemorrhages and intravasal coagulation. In Dengue fever, this form is becoming more and more frequent and is called Dengue hemorrhagic fever (DHF) or Dengue shock syndrome (DSS) depending on the predominant characteristics.
Table 1 Overview of the Most Important Flaviviruses (arthropodborne)
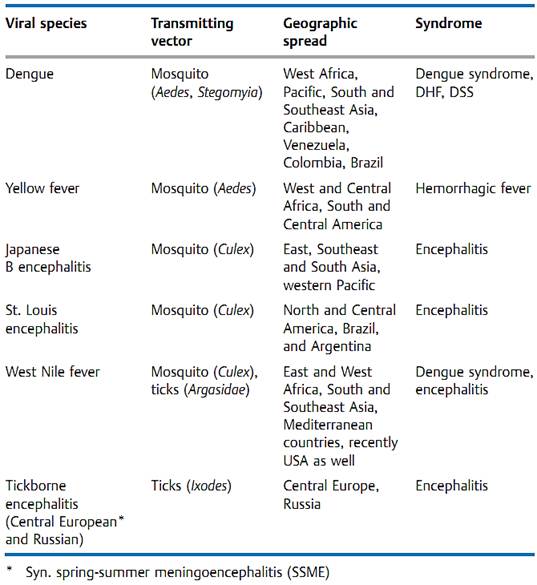
Diagnosis. A flavivirus infection always involves viremia (transmission by bloodsucking arthropods!). The viruses can be isolated from blood by inoculating cell cultures or newborn mice. In autopsies of fatal cases they can be isolated from liver tissue. The viruses are labile by nature and identification can take time, for which reason the diagnostic focus is on serology (titer rise or IgM detection).
Epidemiology and prevention. A cycle of infection involving a vertebrate host (mammals, birds) and a transmitting vector (bloodsucking mosquitoes and flies, ticks) has developed for most flavivirus infections. The cycles are efficient for the virus and relatively harmless for the host. The vertebrate host frequently shows few signs of disease and recovers from the infection after a brief viremia. During this period, the bloodsucking vector is infected, which thereafter remains a lifelong salivary secretor and thus infectious. In ticks, transovarian transmission of the virus is also possible. The human host is a dead end for the virus, not a normal component of the cycle. Exceptions to this are Dengue fever and urban yellow fever.
Humans are the only known main hosts for the Dengue virus. There are two forms of yellow fever: rural or jungle (“sylvatic”) yellow fever with a monkey-mosquito-monkey (sometimes human) cycle andurbanyellow fever with humans as the main hosts and Aedes mosquitoes as the transmitting vectors. This form is on the upswing due to increasing numbers of breeding places (e.g., empty tin cans in garbage piles) for the vector. Another “new” (more accurately: revived) infectious disease is the West Nile viral infection,
Hepaciviruses (Hepatitis C and G)
Pathogen. A series of hepatitis infections following blood transfusions was observed that could not be identified as either hepatitis A or hepatitis B and were therefore designated as “non-A-non-B (NANB) hepatitis."
The discovery of the hepatitis C virus (HCV) by molecular biological means in 1988 was an elegant piece of work: RNA was extracted from the plasma of an infected chimpanzee, from which cDNA was produced using reverse transcriptase. The cDNA was then cloned and the corresponding proteins expressed. About one million clones were tested for reactivity with sera from patients suffering from chronic NANB hepatitis. A protein was found by this method that reacted with antibodies to NANB, whereupon the corresponding cloned DNA was used as a probe to identify further overlapping gene segments. They belong to a flavivirus with approximately 10 kb sense RNA and several genotypes. A similar strategy led to identification of a further flavivirus that also causes hepatitis, now known as the hepatitis G virus (HGV).
Pathogenesis and clinical picture. Hepatitis C resembles hepatitis B in many respects. One major difference is that it much more frequently produces a persistent infection (85%) and, in 70% of cases, develops into a chronic hepatitis, resulting in cirrhosis of the liver within 20 years and a hepatocellular 8 carcinoma (HCC) in a further 10 years. The reason for the high level of viral persistence is thought to be a pronounced mutability facilitating evasion of the immune defenses (quasispecies of RNA viruses).
Diagnosis of hepatitis C is done with antibody EIA using genetically engineered viral proteins. Western blot can be used to confirm the result. The RNA can be detected by means of RT-PCR and the course of therapy can be monitored with quantitative PCR.
Epidemiology and prevention. The incidence of HCV in Europe is about 0.3%, with a decreasing tendency in the younger segment of the population.
About 50% of acute hepatitis cases are HCV infections. Transmission is by blood and blood products. High-risk persons include dialysis patients, healthcare staff, and needle-sharing drug consumers. Perinatal transmission is possible, but sexual contact does not appear to be a risk factor. The transmission route is not apparent in many cases, giving rise to the expression “community-acquired infection.” Feasible protective measures are the same as in hepatitis B; no immunization by vaccine is available. Especially in combination with ribavirin, therapeutic use of interferon can lead to elimination of the virus in persistent infections and thus to prevention of cirrhosis of the liver and HCC.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|