


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 18-10-2021
Date: 18-11-2021
Date: 5-9-2021
|
Adenylyl Cyclase
The recognition of a chemical signal by some GPCR, such as the β- and α2-adrenergic receptors, triggers either an increase or a decrease in the activity of adenylyl cyclase (AC). This is a membrane-bound enzyme that converts ATP to 3ʹ,5ʹ-adenosine monophosphate (cyclic AMP, or cAMP). The chemical signals are most often hormones or neurotransmitters, each of which binds to a unique type of GPCR. Therefore, tissues that respond to more than one signal must have several different GPCR, each of which can be linked to AC.
1. Guanosine triphosphate–dependent regulatory proteins: The effect of the activated, occupied GPCR on second messenger formation is indirect, mediated by specialized trimeric proteins (α, β, and γ subunits) of the cell membrane. These proteins, referred to as G proteins because the α subunit binds guanosine di- or triphosphates (GDP or GTP), form a link in the chain of communication between the receptor and AC. In the inactive form of a G protein, the α subunit is bound to GDP (Fig. 1).
Ligand binding causes a conformational change in the receptor, triggering replacement of this GDP with GTP. The GTP-bound form of the α subunit dissociates from the βγ subunits and moves to AC, affecting enzyme activity. Many molecules of active Gα protein are formed by one activated receptor. [Note: The ability of a hormone or neurotransmitter to stimulate or inhibit AC depends on the type of Gα protein that is linked to the receptor. One type, designated Gs, stimulates AC (see Fig. 3), whereas another type, designated Gi, inhibits the enzyme (not shown).]
The actions of the Gα–GTP complex are short-lived because Gα has an inherent GTPase activity, resulting in the rapid hydrolysis of GTP to GDP. This causes inactivation of Gα, its dissociation from AC, and its reassociation with the βγ dimer.
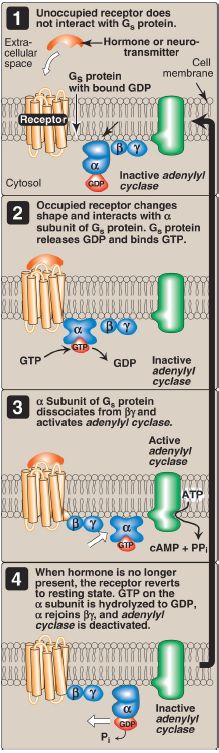
Figure 1: The recognition of chemical signals by certain membrane receptors triggers an increase (or, less often, a decrease) in the activity of adenylyl cyclase. GDP and GTP = guanosine di- and triphosphates; cAMP = cyclic adenosine monophosphate.
Toxins from Vibrio cholerae (cholera) and Bordetella pertussis (whooping cough) cause inappropriate activation of AC through covalent modification (ADP-ribosylation) of different G proteins. With cholera, the GTPase activity of Gαs is inhibited in intestinal cells. With whooping cough, Gαi is inactivated in respiratory tract cells.
2. Protein kinases: The next step in the cAMP second messenger system is the activation of a family of enzymes called cAMP-dependent protein kinases such as protein kinase A (PKA), as shown in Figure 2. cAMP activates PKA by binding to its two regulatory subunits, causing the release of its two catalytically active subunits. These subunits transfer phosphate from ATP to specific serine or threonine residues of protein substrates. The phosphorylated proteins may act directly on the cell’s ion channels or, if enzymes, may become activated or inhibited. [Note: Several types of protein kinases are not cAMP dependent, for example, protein kinase C]
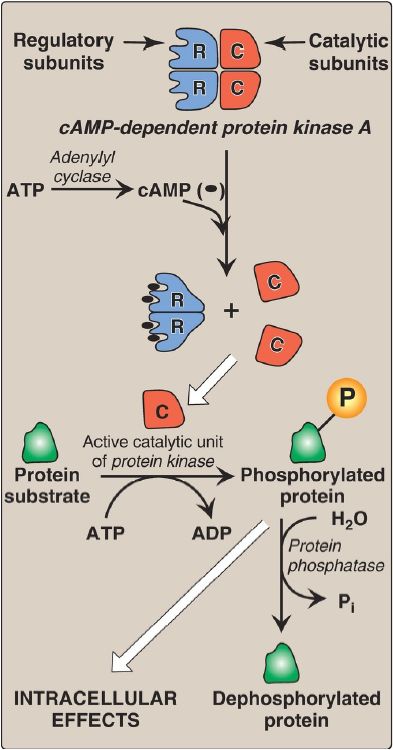
Figure 2: Actions of cyclic adenosine monophosphate (cAMP). = phosphate; ADP = adenosine diphosphate; Pi = inorganic phosphate.
3. Protein phosphatases: The phosphate groups added to proteins by protein kinases are removed by protein phosphatases, enzymes that hydrolytically cleave phosphate esters (see Fig. 2). This insures that changes in protein activity induced by phosphorylation are not permanent.
4. cAMP hydrolysis: cAMP is rapidly hydrolyzed to 5ʹ-AMP by cAMP phosphodiesterase that cleaves the cyclic 3ʹ,5ʹ-phosphodiester bond. 5ʹ- AMP is not an intracellular signaling molecule. Therefore, the effects of neurotransmitter- or hormone-mediated increases of cAMP are rapidly terminated if the extracellular signal is removed. [Note: cAMP phosphodiesterase is inhibited by caffeine, a methylxanthine derivative.]



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
خدمات متعددة يقدمها قسم الشؤون الخدمية للزائرين
|
|
|