


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 3-1-2016
Date: 3-5-2021
Date: 7-5-2021
|
The lac Operon Is Negative Inducible
KEY CONCEPTS
- Transcription of the lacZYA operon is controlled by a repressor protein that binds to an operator that overlaps the promoter at the start of the cluster.
- In the absence of β-galactosides, the lac operon is expressed only at a very low (basal) level.
- The repressor protein is a tetramer of identical subunits coded by the lacI gene.
- β-galactoside sugars, the substrates of the lac operon, are its inducer.
- Addition of specific β-galactosides induces transcription of all three genes of the lac operon.
- The lac mRNA is extremely unstable; as a result, induction can be rapidly reversed.
Structural genes can be distinguished from regulator genes based on the effects of mutations. A mutation in a structural gene deprives the cell of the particular protein for which the gene codes. A mutation in a regulator gene, however, influences the expression of all the structural genes connected to it in cis. The consequences of a regulatory mutation reveal the type of regulation.
Transcription of the lacZYA genes is controlled by a regulator protein encoded by the lacI gene. Although adjacent to the structural genes, lacI comprises an independent transcription unit with its own promoter and terminator. In principle, lacI need not be located near the structural genes because it specifies a diffusible product. The lacI gene can function equally well if moved elsewhere, or it can be carried on a separate DNA molecule (the classic test for a trans-acting regulator).
The lacZYA genes are negatively regulated: They are transcribed unless turned off by the regulator protein. Note that repression is not an absolute phenomenon; turning off a gene is not like turning off a lightbulb. Repression can often be a reduction in transcription by 5- or 100-fold. A mutation that inactivates the regulator causes the structural genes to be continually expressed, a condition called constitutive expression. The product of lacI is called the lac repressor, because its function is to prevent the expression of the lacZYA structural genes.
The lac repressor is a tetramer of identical subunits of 38 kD each. A wild-type cell contains approximately 10 tetramers. The repressor gene is not controlled; it is an unregulated gene. It is transcribed into a monocistronic mRNA at a rate that appears to be governed simply by the affinity of its (poor) promoter for RNA polymerase. In addition, lacI is transcribed into a poor mRNA. This is a common way to restrict the amount of protein made. In this case, the mRNA has virtually no 5′ untranslated region (UTR), which restricts the ability of a ribosome to start translation. These two features account for the low abundance of lac repressor protein in the cell.
The repressor functions by binding to an operator (formally denoted Olac ) at the start of the lacZYA cluster. The sequence of the operator includes an inverted repeat. The operator lies between the promoter (Plac ) and the structural genes (lacZYA). When the repressor binds at the operator, it prevents RNA polymerase from initiating transcription at the promoter. FIGURE 1 expands our view of the region at the start of the lac structural genes. The operator extends from position −5 just upstream of the mRNA start point to position +21 within the transcription unit; thus it overlaps the 3′, right end of the promoter. A mutation that inactivates the operator also causes constitutive expression.
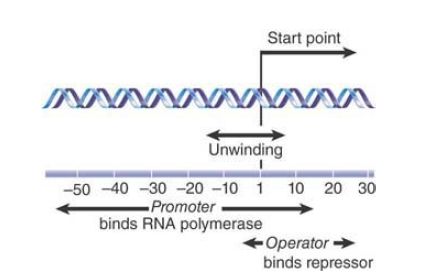
FIGURE 1.The lac repressor and RNA polymerase bind at sites that overlap around the transcription start point of the lac operon.
When cells of E. coli are grown in the absence of a β-galactoside they have no need for β-galactosidase, and they contain very few molecules of the enzyme, about five per cell. When a suitable substrate is added, the enzyme activity appears very rapidly in the bacteria. Within 2 to 3 minutes some enzyme is present, and soon each bacterium accumulates approximately 5,000 molecules of enzyme. (Under suitable conditions, β-galactosidase can account for 5% to 10% of the total soluble protein of the bacterium.) If the substrate is removed from the medium, the synthesis of the enzyme stops as rapidly as it started.
FIGURE 2 summarizes the essential features of this induction. Control of transcription of the lac operon responds very rapidly to the inducer, as shown in the upper part of the figure. In the
absence of inducer, the operon is transcribed at a very low basal level (this is an important concept; see the next section, lac Repressor Is Controlled by a Small-Molecule Inducer). Transcription is stimulated as soon as inducer is added; the amount of lac mRNA increases rapidly to an induced level that reflects a balance between synthesis and degradation of the mRNA.

FIGURE 2. Addition of the inducer results in rapid induction of lac mRNA and is followed after a short lag by synthesis of the enzymes; removal of the inducer is followed by rapid cessation of synthesis.
The lac mRNA (as most mRNA is in bacteria) is extremely unstable and decays with a half-life of only about 3 minutes. This feature allows induction to be reversed rapidly by repressing transcription as soon as the inducer is removed. In a very short time all the lac mRNA is destroyed and enzyme synthesis ceases.
The production of protein is followed in the lower part of the figure. Translation of the lac mRNA produces β-galactosidase (and the products of the other lac genes). A short lag occurs between the appearance of lac mRNA and the appearance of the first completed enzyme molecules (about 2 minutes lapse between the rise of mRNA from basal level and increased protein level). A similar lag occurs between reaching maximal induced levels of mRNA and protein. When the inducer is removed, synthesis of the enzyme ceases almost immediately (as the lacZYA mRNA is quickly degraded), but the β-galactosidase in the cell is more stable; thus the enzyme activity remains at the induced level for longer.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|