


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2-5-2016
Date: 27-4-2016
Date: 16-5-2021
|
tRNAs Are Charged with Amino Acids by Aminoacyl-tRNA Synthetases
KEY CONCEPTS
- Aminoacyl-tRNA synthetases are a family of enzymes that attach amino acid to tRNA, generating aminoacyltRNA in a two-step reaction that uses energy from ATP.
- Each tRNA synthetase aminoacylates all the tRNAs in an isoaccepting group, representing a particular amino acid.
- Recognition of a tRNA is based on a particular set of nucleotides, the tRNA “identity set”; these nucleotides often are concentrated in the acceptor-stem and anticodon-loop regions of the molecule.
It is necessary for tRNAs to have certain characteristics in common but yet be distinguished by others. The crucial feature that confers this capacity is the ability of tRNA to fold into a specific tertiary structure. Changes in the details of this structure, such as the angle of the two arms of the “L” or the protrusion of individual bases, may distinguish the individual tRNAs.
All tRNAs can fit in the P and A sites of the ribosome. At one end they are associated with mRNA via codon–anticodon pairing, and at the other end the polypeptide is being synthesized and transferred.
Similarly, all tRNAs (except the initiator) share the ability to be recognized by elongation factors (EF-Tu or eEF1) for binding to the ribosome. The initiator tRNA is recognized instead by IF-2 or eIF2. Thus, the tRNA set must possess common features for interaction with elongation factors and for identification of the tRNA initiator. Amino acids enter the translation pathway through the action of aminoacyl-tRNA synthetases, which provide the essential decoding step converting the information in nucleic acids into the polypeptide sequence. All synthetases function by the mechanism depicted in FIGURE 1:
- The amino acid first reacts with ATP to form an aminoacyladenylate intermediate, releasing pyrophosphate. Part of the energy released in ATP hydrolysis is trapped as a high-energy mixed anhydride linkage in the adenylate.
- Next, either the 2′–OH or 3′–OH group located on the 3′-A76 nucleotide of tRNA attacks the carbonyl carbon atom of the mixed anhydride, generating aminoacyl-tRNA with concomitant release of AMP. (Note that key conserved nucleotides of tRNAs are always given the same name for consistency. Thus, the terminal nucleotide of every tRNA is called A76, even when the length of a given tRNA may vary from that typical length.)
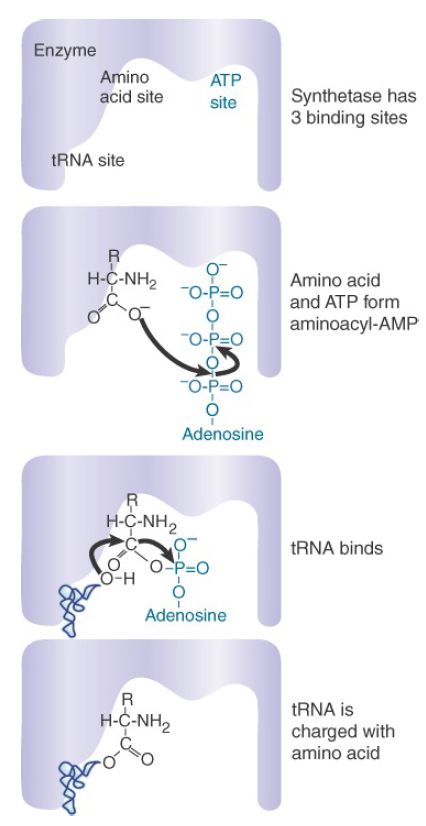
FIGURE 1. An aminoacyl-tRNA synthetase charges tRNA with an amino acid.
A subset of four tRNA synthetases—those specific to glutamine, glutamate, arginine, and lysine—require the presence of tRNA to synthesize the aminoacyl-adenylate intermediate. For these enzymes, the tRNA synthetase is properly considered as a ribonucleoprotein particle (RNP), in which the RNA subunit functions to assist the protein in attaining a catalytically competent conformation. In the second step of aminoacylation, the amino acid portion of the aminoacyl adenylate is then transferred to the RNA component of the RNP (i.e., the tRNA).
Each tRNA synthetase is selective for a single amino acid among all the amino acids in the cellular pool. It also discriminates among all tRNAs in the cell. Usually, each amino acid is represented by more than one tRNA. Several tRNAs may be needed to recognize synonymous codons, and sometimes multiple types of tRNA base pair with the same codon. Multiple tRNAs representing the same amino acid are called isoaccepting tRNAs; because they are all recognized by the same synthetase, they are also described as its cognate tRNAs.
All tRNAs possess the canonical L-shaped tertiary structure . The tRNA folds such that the acceptor and T stems form one coaxial stack, while the D and anticodon stems together form the perpendicular arm of the L-shape. The anticodon loop and CCA acceptor end are located at opposite ends of the molecule and are separated by approximately 40 Å. The globular hinge region of the tRNA, which connects the two perpendicular stacks, is composed of the D-loop, T-loop, variable arm, and two-nucleotide spacer between the acceptor and D stems. Most tRNAs possess small variable regions consisting of a four- to five-nucleotide loop, whereas a few isoaccepting groups feature a larger variable arm including a base-paired stem, which protrudes from the globular core. The common tRNA L-shape is essential for the interaction of all tRNAs with elongation factors and with the ribosome.
Within the context of this common L-shaped structure, enforced by the presence of conserved tertiary interactions within the globular core, tRNA sequences are found to diverge at a majority of positions in all four arms of the molecule. This sequence diversity can generate subtle differences in the angle between the two arms of the L-shape and, more important, leads to variations in the detailed path of the polynucleotide backbone throughout the molecule. It is this structural diversity that forms the basis for discrimination by the tRNA synthetases.
tRNA synthetases discriminate among tRNAs by means of two general mechanisms: direct readout and indirect readout. In direct readout, the enzyme recognizes base-specific functional groups directly; for example, a surface amino acid of a tRNA synthetase may accept a hydrogen bond from the exocyclic amine group of guanine (the N2 of G), a minor-groove group not found on the other three bases. By contrast, in indirect readout, the enzyme directly binds nonspecific portions of the tRNA: the sugar–phosphate backbone and nonspecific portions of the nucleotide bases. For example, sequences in the variable and D arms of a tRNA may produce a distinctively shaped surface that is complementary to the cognate tRNA synthetase, but not to other tRNA synthetases. In this way nucleotides distant from the enzyme–tRNA interface create an interface structure that is, in turn, directly bound. Both direct and indirect readout usually function within the context of mutual induced fit: Conformational changes in both the tRNA and enzyme occur after initial binding to form a productive catalytic
complex. Both these mechanisms also often involve the participation of bound water molecules at the interface between the tRNA and enzyme. For example, when glutaminyl-tRNA synthetase (GlnRS) binds tRNAGln , two domains of the enzyme rotate with respect to each other; simultaneously, the 3′–single-stranded end and the anticodon loop of the tRNA undergo substantial conformational changes as compared with their presumed structures in the unliganded state.
In many cases the determinants in tRNA that are needed for specific recognition are located at the extremities of the molecule, in the acceptor stem and the anticodon loop. However, examples exist where nucleotides in the tertiary core provide the identity signals. Another commonly used identity nucleotide is the “discriminator base” at homologous position 73 in the tRNA, which is located directly 5′ to the 3′-terminal CCA sequence. Interestingly, the anticodon sequence of the tRNA is not necessarily required for specific tRNA synthetase recognition. In general, the tRNA identity set is idiosyncratic to each tRNA synthetase.
The identity determinants vary in their importance and are sometimes conserved in evolution. The conservation in tRNA identity elements is demonstrated by the capacities of many tRNA synthetases to aminoacylate tRNAs that are derived from different organisms. Hypotheses regarding the set of tRNA identity elements necessary for selection by a tRNA synthetase are derived from Xray cocrystal structures of tRNA synthetase complexes, from classical genetics, and from in vitro mutagenesis. Final proof that a tRNA identity set has been well defined is obtained from transplantation experiments, in which the hypothesized set of nucleotides is incorporated into a tRNA from a different isoaccepting group. For example, replacement of 15 nucleotides in the acceptor stem and anticodon loop of tRNAAsp , with the corresponding nucleotides in tRNAGln , allowed glutaminyl-tRNA synthetase (GlnRS) to aminoacylate the modified tRNAAsp with glutamine, with an efficiency and selectivity comparable to that of the cognate GlnRS reaction.
Many tRNA synthetases can specifically aminoacylate a tRNA “minihelix,” which consists only of the acceptor and TψC arms of the molecule. In some cases, a tRNA microhelix, consisting of the acceptor stem alone closed at its distal end by a stable tetraloop, can serve as a substrate. For both minihelices and microhelices, the efficiency of aminoacylation is substantially weaker than in the case of the intact tRNA. However, these experiments have some significance to the evolutionary development of tRNA synthetase complexes. At an early evolutionary stage, tRNAs may have consisted solely of the acceptor arm of the contemporary molecule.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|