


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Quality Control of mRNA Translation Is Performed by Cytoplasmic Surveillance Systems |
|
|
|
Read More
Date: 22-4-2021
Date: 13-12-2015
Date: 14-12-2015
|
Quality Control of mRNA Translation Is Performed by Cytoplasmic Surveillance Systems
KEY CONCEPTS
- Nonsense-mediated decay (NMD) targets mRNAs with premature stop codons.
- Targeting of NMD substrates requires a conserved set of UPF and SMG proteins.
- Recognition of a termination codon as premature involves unusual 3′ UTR structure or length in many organisms and the presence of downstream exon junction complexes (EJCs) in mammals.
- Nonstop decay (NSD) targets mRNAs lacking an inframe termination codon and requires a conserved set of SKI proteins.
- No-go decay (NGD) targets mRNAs with stalled ribosomes in their coding regions.
Some kinds of mRNA defects can be assessed only during translation. Surveillance systems have evolved to detect three types of mRNA defects that threaten translational fidelity and to target the defective mRNAs for rapid degradation. FIGURE 1 shows the substrates for each of these three systems. All three systems involve abnormal translation termination events, so it is useful to review what happens during normal termination . When a translating ribosome reaches the termination (stop) codon, a pair of release factors (eRF1 and eRF2 in eukaryotes) enters the ribosomal A site, which is normally filled by incoming tRNAs during elongation. The release factor complex mediates the release of the completed polypeptide, followed by the mRNA, remaining tRNA, and ribosomal subunits.

FIGURE 1. Substrates for cytoplasmic surveillance systems. Nonsense-mediated decay (NMD) degrades mRNAs with a premature termination codon (PTC) position ahead of its normal termination codon (TC). Nonstop decay (NSD) degrades mRNAs lacking an in-frame termination codon. No-go decay (NGD) degrades mRNAs having ribosome stalled in the coding region.
Nonsense-mediated decay (NMD) targets mRNAs containing a premature termination codon (PTC). Its name comes from nonsense mutation, which is only one way that mRNAs with a PTC can be generated. Genes without nonsense mutations can give rise to aberrant transcripts containing a PTC by (1) RNA polymerase error or (2) incomplete, incorrect, or alternative splicing. It has been estimated that almost half of alternatively spliced pre-mRNAs generate at least one form with PTC. About 30% of known disease-causing alleles probably encode an mRNA with a PTC. An mRNA with a PTC will produce C-terminal truncated polypeptides, which are considered to be particularly toxic to a cell due to their tendency to trap multiple binding partners in nonfunctional complexes. The NMD pathway has been found in all eukaryotes. Targeting of PTC-containing mRNAs requires translation and a conserved set of protein factors. They include three Upf proteins (Upf1, Upf2, and Upf3) and four additional proteins (Smg1, Smg5, Smg 6, and Smg7). Upf1 is the first NMD protein to act, binding to the terminating ribosome—specifically to its release factor complex. UPF attachment tags the mRNA for rapid decay. The specific roles of the NMD factors have not yet been defined, although phosphorylation of ribosome-bound Upf1 by Smg1 is critical. Their combined actions condemn the mRNA to the general decay machinery and stimulate rapid deadenylation. The target mRNAs are degraded by both 5′ to 3′ and 3′ to 5′ pathways.
How are PTCs distinguished from the normal termination codon further downstream? The mechanism has been studied extensively both in yeast and in mammalian cells, where it is somewhat different; these mechanisms are illustrated in FIGURE 2. The major signal that identifies a PTC in mammalian cells is the presence of a splice junction, marked by an exon junction complex (EJC) downstream of the premature termination codon. The majority of genes in higher eukaryotes do not have an intron interrupting the 3′ UTR, so authentic termination codons are not generally followed by a splice junction. During the pioneer round of translation for a normal mRNA, all EJCs occur within the coding region and are displaced by the transiting ribosome. During the pioneer round of translation for an NMD substrate, Upf2 and Upf3 proteins bind to the residual downstream EJC(s), targeting it for degradation.
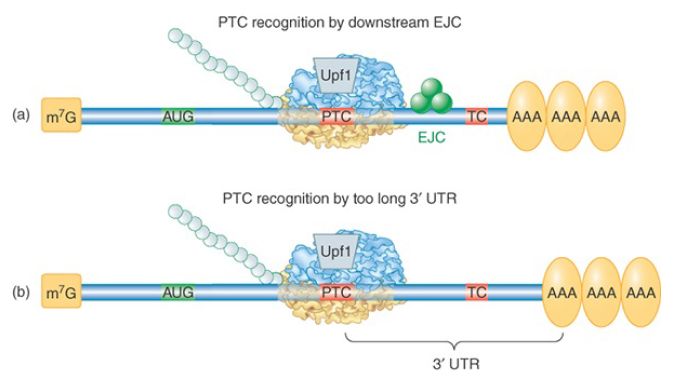
FIGURE 2. Two mechanisms by which a termination codon is recognized as premature. (a) In mammals, the presence of an EJC downstream of a termination codon targets the mRNA for NMD. (b) In probably all eukaryotes, an abnormally long 3′ UTR is recognized by the distance between the termination codon and the poly(A)– PABP complex. In either case, the Upf1 protein binds to the terminating ribosome to trigger decay.
Most S. cerevisiae genes are not interrupted by introns at all, so the mechanism for PTC detection must be different. In this case an abnormally long 3′ UTR is the warning sign. This was demonstrated by the finding that extension of the 3′ UTR of a normal mRNA could convert it into a substrate for NMD. A current model proposes that proper translation termination at a stop codon requires a signal from a nearby PABP. Although 3′ UTRs are highly variable in nucleotide length, the physical distance between the termination codon and the poly(A) tail is not strictly a function of length because secondary structures and interactions between bound RBPs can compress the distance. The requirement for PABP was demonstrated in multiple organisms by tethering a PABP close to the PTC, as illustrated in FIGURE 3. The mRNA was no longer targeted by NMD. PTC recognition also occurs independently of splicing in Drosophila, Caenorhabditis elegans, plants, and in some mammalian mRNAs, suggesting that the length and structure of the 3′ UTR may be critical for the normal process of translation termination in all eukaryotic organisms.
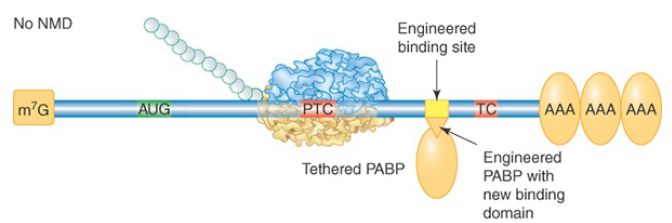
FIGURE 3. Effect of tethering a PABP near a premature termination codon. A PABP gene was altered to express a phage RNA-binding domain. Its binding site was engineered into a test NMD-substrate gene. The tethered PABP prevented the usual rapid degradation of this mRNA by NMD. This method has many applications in molecular biology.
Some normal mRNAs are targeted by NMD. These were identified by experiments in which Upf1 levels were reduced, resulting in a subset of transcripts that increased in abundance. The list of normal NMD substrates includes mRNAs with especially long 3′ UTRs, mRNAs encoding selenoproteins (which use the termination codon UGA as a selenocysteine codon), and an unknown number of alternatively spliced mRNAs. Not all targeted mRNAs are predicted to be NMD substrates based on our current understanding. NMD may turn out to be an important rapid decay pathway for a variety of short-lived mRNAs.
Bacteria are also able to rapidly degrade mRNAs with premature termination codons. In the E. coli version of NMD, the endonuclease RNase E cuts the mRNA in the region 3′ to the PTC, which is in an abnormally unprotected state due to premature release of ribosomes. This mechanism probably does not require any additional means to distinguish a PTC from the correct termination codon and would also work for polycistronic mRNAs. Nonstop decay (NSD) targets mRNAs that lack an in-frame termination codon . Failure to terminate results in a ribosome translating into the poly(A) tail and probably stalling at the 3′ end. NSD substrates are generated mainly by premature transcription termination and polyadenylation in the nucleus. Such prematurely polyadenylated transcripts are surprisingly common. Analysis of random cDNA populations derived from yeast and human mRNAs suggests that 5% to 10% of
polyadenylation events may occur at upstream “cryptic” sites that resemble an authentic polyadenylation signal. Targeting nonstop substrates involves a set of factors called the SKI proteins. The ribosome is released from the mRNA by the action of Ski7. Ski7 has a GTPase domain similar to eEF3 and probably binds to the ribosome in the A site to stimulate release. The subsequent recruitment of the other SKI proteins and the exosome results in 3′ to 5′ decay of the mRNA. Decay of nonstop substrates can also occur in the absence of Ski7 and proceeds by decapping and 5′ to 3′ digestion. Susceptibility to decapping could be due to the pioneer ribosome displacing PABPs as it traverses the poly(A) tail. Rapid decay of nonstop substrates results in not only prevention of toxic polypeptides but also liberation of trapped ribosomes. Interestingly, E. coli uses a specialized noncoding RNA (tmRNA) that acts like both a tRNA and an mRNA to rescue ribosomes stalled on a nonstop mRNA. tmRNA directs the addition of a short peptide that targets the defective protein product for degradation, provides a stop codon to allow recycling of the ribosome, and targets degradation of the defective mRNA by RNAse R.
No-go decay (NGD) targets mRNAs with ribosomes stalled in the coding region codon . Transient or prolonged stalling can be caused by natural features of some mRNAs, including strong secondary structures and rarely used codons (whose cognate tRNAs are in low abundance). This newly discovered surveillance pathway has been studied only in yeast and is the least understood of the three. Targeting of the mRNA involves recruitment of two proteins, Dom34 and Hbs1, which are homologous to eRF1 and eRF3, respectively. mRNA degradation is initiated by an endonucleolytic cut, and the 5′ and 3′ fragments are digested by the exosome and Xrn1. Dom34 might be the endonuclease, as one of its domains is nuclease-like. Why would a normal mRNA have hard-to-translate sequences that might condemn it to rapid degradation? Such sequences can be thought of as another kind of destabilizing element. Evolutionary retention of impediments to efficient translation suggests that they serve an important function in controlling the half-life of these mRNAs.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|