


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Ig Genes Are Assembled from Discrete DNA Segments in B Lymphocytes |
|
|
|
Read More
Date: 8-5-2016
Date: 29-12-2015
Date: 25-12-2015
|
Ig Genes Are Assembled from Discrete DNA Segments in B Lymphocytes
KEY CONCEPTS
- An antibody consists of a tetramer of two identical light (L) chains and two identical heavy (H) chains. There are two families of L chains (λ and κ) and a single family of H chains.
- Each chain has an N-terminal variable (V) region and a C-terminal constant (C) region. The V region recognizes the antigen, and the C region mediates the effector response. V and C regions are separately encoded by V(D)J gene segments and C gene segments.
- A gene coding for a whole Ig chain is generated by somatic recombination of V(D)J genes (variable, diversity, and joining genes in the H chain; variable and joining genes in the L chain) giving rise to V domains, to be expressed together with a given C gene (C domain).
Sophisticated evolutionary mechanisms have evolved to guarantee that the organism is prepared to produce specific antibodies for a broad variety of naturally occurring and manmade components that it has never encountered before. Each antibody is a tetramer consisting of two identical immunoglobulin light (L) chains and two identical immunoglobulin heavy (H) chains (FIGURE 1). Humans and mice have two types of L chains (λ and κ) and nine types of H chains. The class is determined by the H chain constant (C) region, which mediates the antibody’s biological effector functions.
Different Ig classes have different effector functions. L chains and H chains share the same general type of organization in that each protein chain consists of two principal domains: the N-terminal variable (V) region and the C-terminal constant (C) region.
These were defined originally by comparing the amino acid sequences of different Ig chains secreted by monoclonal B cell tumors (plasmacytomas). As the names suggest, the V regions show considerable changes in sequence from one protein to the next, whereas the C regions show substantial homology.
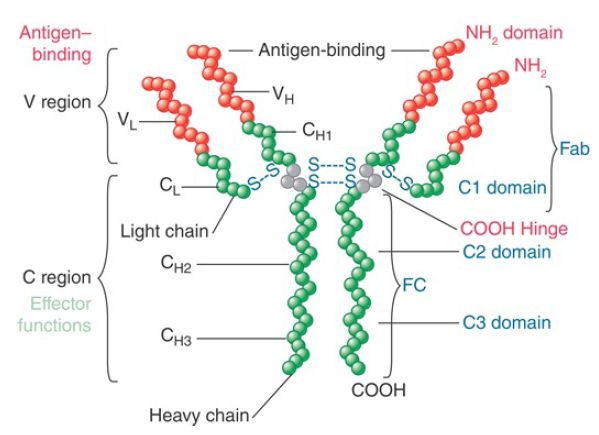
FIGURE 1. An antibody (immunoglobulin, or Ig) molecule is a heterodimer consisting of two identical heavy chains and two identical light chains. Schematized here is an IgG1, which comprises an N-terminal variable (V) region and a C-terminal constant (C) region.
Corresponding regions of the L and H chains associate to generate distinct domains in the Ig protein. The V domain is generated by association between a recombined H chain V DJ segment and a recombined L chain Vλ Jλ or Vk Jk segment. The V domain is responsible for recognizing the antigen. Generation of V domains of different specificities creates the ability to respond to diverse antigens. The total number of V region genes for either L or H chain proteins is measured in hundreds. Thus, an antibody displays the maximum versatility in the region responsible for binding the antigen. The C regions in the subunits of the Ig tetramer associate to generate individual C domains. The first domain results from association of the single C region of the L chain (CL) with the CH1 domain of the H chain C region (CH ). The two copies of this domain complete the arms of the Y-shaped antibody molecule. Association between the C regions of the H chains generates the remaining C domains, which vary in number (three of four) depending on the type of H chain.
Many genes encode V regions, but only a few genes encode C regions. In this context, “gene” means a sequence of DNA coding for a discrete part of the final Ig polypeptide (H or L chain). Thus, recombined V(D)J genes encode variable regions, and C genes encode constant regions. To construct a unit that can be expressed in the form of a whole L or H chain, a V(D)J gene must be joined physically to a C gene.
The sequences encoding L chains and H chains are assembled in the same way: Any one of several V(D)J gene segments may be joined to any one of a few C gene segments. This somatic DNA recombination occurs in the B lymphocyte in which the BCR/antibody is expressed. The large number of available V(D)J gene segments is responsible for a major part of the diversity of Igs. Not all diversity is encoded in the genome, though; more is generated by changes that occur during the assembly process of a functional gene.
Essentially the same mechanisms underlie the generation of functional genes encoding the protein chains of the TCR. Two types of receptor are found on T cells—one consisting of α and β chains, and the other consisting of γ and δ chains. Like the genes encoding Igs, the genes encoding the individual chains in TCRs consist of separate parts, including recombined V(D)J gene segments and C region genes.
The organism does not possess the functional genes in the germline for producing a particular BCR or TCR. It possesses a large repertoire of V gene segments and a smaller number of C gene segments. The subsequent assembly of a productive gene from these parts allows the BCR/TCR to be expressed on B and T cells so that it is available to react with the antigen. V(D)J DNA rearrangement occurs before exposure to antigen. Productive V(D)J rearrangements are expressed by B cells and T cells as surface BCRs and TCRs, which provide the structural substrate for selection of those clones capable of binding the antigen. The arrangement of V(D)J gene segments and C gene segments is different in the cells expressing BCR or TCR from all other somatic cells or germ cells. The entire process occurs in somatic cells and does not affect the germline; thus, the progeny of the organism does not inherit the specific response to an antigen.
The Ig κ and λ chains and H chain loci reside on different chromosomes, and each locus consists of its own set of both V gene segments and C gene segments. This germline organization is found in the germline and in the somatic cells of all lineages. In a B cell expressing an antibody, though, each chain—one L type (either κ or λ) and one H type—is encoded by a single intact DNA sequence. The recombination event that brings a V(D)J gene segment in proximity to, and to be expressed with, a C gene segment creates a productive gene consisting of exons that correspond precisely with the functional domains of the protein.
After transcription of the whole DNA sequence into a primary RNA transcript, the intronic sequences are removed by RNA splicing. V(D)J recombination occurs in developing B lymphocytes. A B lymphocyte, in general, carries only one productive rearrangement of L chain gene segments (either κ or λ) and one of H chain gene segments. Likewise, a T lymphocyte productively rearranges an α gene and a β gene or a δ gene and a γ gene. The BCR and TCR expressed by any one cell is determined by the particular configuration of V gene segments and C gene segments that have been joined.
The principles by which functional genes are assembled are the same in each family, but there are differences in the details of the organization of both the V and C gene segments, and correspondingly of the recombination reaction between them. In addition to these segments, other short DNA sequences (D segments and J, “joining,” segments) are included in the functional somatic loci.
If any L chain can pair with any H chain, about 106 different L chains and about 106 different H chains can pair to generate more than 1012 different Igs. Indeed, a mammal has the ability to generate 1012 or more different antibody specificities.



|
|
|
|
دور في الحماية من السرطان.. يجب تناول لبن الزبادي يوميا
|
|
|
|
|
|
|
العلماء الروس يطورون مسيرة لمراقبة حرائق الغابات
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|