


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 12-5-2021
Date: 28-10-2020
Date: 23-3-2021
|
Nucleic Acids Hybridize by Base Pairing
KEY CONCEPTS
-Heating causes the two strands of a DNA duplex to separate.
-The Tm is the midpoint of the temperature range for denaturation.
-Complementary single strands can renature when the temperature is reduced.
-Denaturation and renaturation/hybridization can occur with DNA–DNA, DNA–RNA, or RNA–RNA combinations and can be intermolecular or intramolecular.
-The ability of two single-stranded nucleic acids to hybridize is a measure of their complementarity.
A crucial property of the double helix is the capacity to separate the two strands without disrupting the covalent bonds that form the polynucleotides and at the (very rapid) rates needed to sustain genetic functions. The specificity of the processes of denaturation and renaturation is determined by complementary base pairing.
The concept of base pairing is central to all processes involving nucleic acids. Disruption of the base pairs is crucial to the function of a double-stranded nucleic acid, whereas the ability to form base pairs is essential for the activity of a single-stranded nucleic acid. FIGURE 1. shows that base pairing enables complementary single-stranded nucleic acids to form a duplex:

FIGURE 1. Base pairing occurs in duplex DNA and also in intraand intermolecular interactions in single-stranded RNA (or DNA).
An intramolecular duplex region can form by base pairing between two complementary sequences that are part of a single-stranded nucleic acid.
A single-stranded nucleic acid can base pair with an independent, complementary single-stranded nucleic acid to form an intermolecular duplex.
Formation of duplex regions from single-stranded nucleic acids is most important for RNA, but it is also important for single-stranded viral DNA genomes. Base pairing between independent
complementary single strands is not restricted to DNA–DNA or RNA–RNA; it also can occur between DNA and RNA.
The lack of covalent bonds between complementary strands makes it possible to manipulate DNA in vitro. The hydrogen bonds that stabilize the double helix are disrupted by heating or by low salt concentration. The two strands of a double helix separate entirely when all of the hydrogen bonds between them are broken.
Denaturation of DNA occurs over a narrow temperature range and results in striking changes in many of its physical properties. The midpoint of the temperature range over which the strands of DNA separate is called the melting temperature (Tm ) and it depends on the G-C content of the duplex. Each G-C base pair has three hydrogen bonds; as a result, it is more stable than an A-T base pair, which has only two hydrogen bonds. The more G-C base pairs in a DNA, the greater the energy that is needed to separate the two strands. In solution under physiological conditions, a DNA that is 40% G-C (a value typical of mammalian genomes) denatures with a Tm of about 87°C, so duplex DNA is stable at the temperature of the cell.
The denaturation of DNA is reversible under appropriate conditions. Renaturation depends on specific base pairing between the complementary strands. FIGURE 2. shows that the reaction takes place in two stages. First, single strands of DNA in the solution encounter one another by chance; if their sequences are complementary, the two strands base pair to generate a short, double-stranded region. This region of base pairing then extends along the molecule, much like a zipper, to form a lengthy duplex.
Complete renaturation restores the properties of the original double helix. The property of renaturation applies to any two complementary nucleic acid sequences. This is sometimes called annealing, but the reaction is more generally called hybridization whenever nucleic acids from different sources are involved, as in the case when DNA hybridizes to RNA. The ability of two nucleic acids to hybridize constitutes a precise test for their complementarity because only complementary sequences can form a duplex.
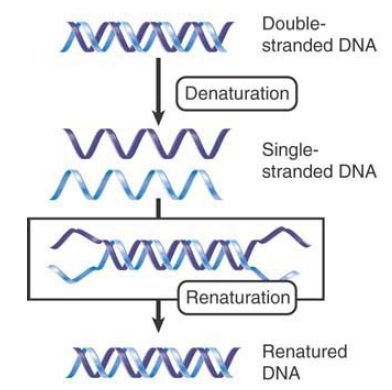
FIGURE 2. Denatured single strands of DNA can renature to give the duplex form.
Experimentally, the hybridization reaction is used to combine two single-stranded nucleic acids in solution and then to measure the amount of double-stranded material that forms. FIGURE 3. illustrates a procedure in which a DNA preparation is denatured and the single strands are linked to a filter. A second denatured DNA (or RNA) preparation is then added. The filter is treated so that the second preparation of nucleic acid can attach to it only if it is able to base-pair with the DNA that was originally linked to the filter.
Usually the second preparation is labeled so that the hybridization reaction can be measured as the amount of label retained by the filter. Alternatively, hybridization in solution can be measured as the change in UV absorbance of a nucleic acid solution at 260 nm as detected via spectrophotometry. As DNA denatures to single strands with increasing temperature, UV absorbance of the DNA solution increases; UV absorbance consequently decreases as ssDNA hybridizes to complementary DNA or RNA with decreasing temperature.
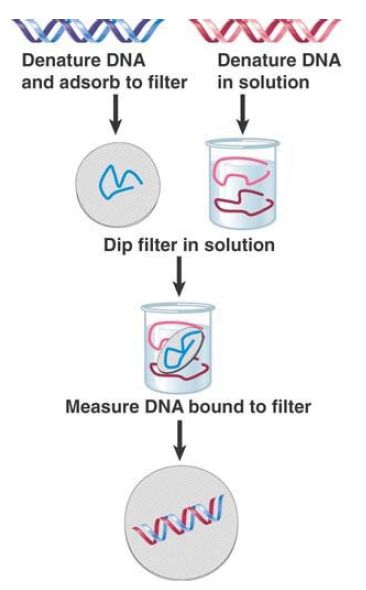
FIGURE 3. Filter hybridization establishes whether a solution of denatured DNA (or RNA) contains sequences complementary to the strands immobilized on the filter.
The extent of hybridization between two single-stranded nucleic acids is determined by their complementarity. Two sequences need not be perfectly complementary to hybridize under the appropriate conditions. If they are similar but not identical, an imperfect duplex is formed in which base pairing is interrupted at positions where the two single strands are not complementary.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|