
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-6-2016
Date: 10-7-2018
Date: 25-5-2017
|
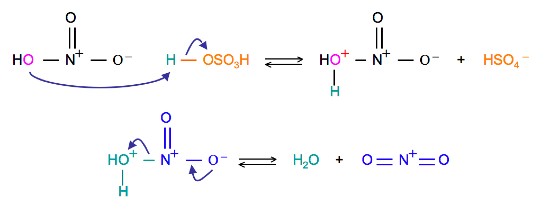
The source of the nitronium ion is through the protonation of nitric acid by sulfuric acid, which causes the loss of a water molecule and formation of a nitronium ion.
The first step in the nitration of benzene is to activate HNO3with sulfuric acid to produce a stronger electrophile, the nitronium ion.
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce Nitrobenzene.
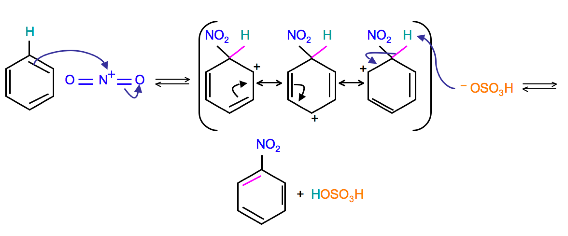
(Resonance forms of the intermediate can be seen in the generalized electrophilic aromatic substitution)



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|