


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-7-2019
Date: 24-9-2020
Date: 19-9-2018
|
Case Study—Racemic and Non-Racemic Drugs
Currently, about 80% of the new drug molecules approved for clinical use are chiral, and of these, the vast majority are developed and marketed as single enantiomers. This was not always the case however, and prior to the 1990s most chiral drug substances were manufactured and used as racemic mixtures (equal mixtures of R and S enantiomers). This might seem surprising since we have already noted that the chiral macromolecules of living organisms can usually distinguish between the enantiomeric forms of drug species. It turns out however that the separation of drug enantiomers is not a trivial task, especially when it comes to manufacturing many tons of a drug. Thus, the current availability of single enantiomer drugs is a result of the development in recent decades of synthetic methods and separation technologies that enable the large-scale manufacture of single-enantiomer drugs. In this case study, we will discuss racemic and non-racemic drug substances by focusing on the specific examples of omeprazole, ibuprofen, ketoprofen, and naproxen.
Omeprazole and Esomeprazole
It can generally be assumed that one enantiomeric form of a drug substance will be more active at a given biological target than the other. In fact, the terms eutomer and distomer are sometimes used to describe, respectively, the “active” and “inactive” enantiomer of a racemic drug substance. Sometimes however, the situation is more complicated, as is the case with the proton pump inhibitor omeprazole. Omeprazole (marketed as Prilosec®) was the first member of a new class of drugs intended to treat gastroesophageal reflux disease by directly inhibiting the proton pump (a H+/K+ ATPase) responsible for secreting protons (H+) into the stomach. As we noted in Section 3.10, omeprazole contains a chirality center at the tetrahedral sulfur atom and thus can exist in two enantiomeric forms (R and S). Omeprazole was originally developed and marketed as a racemic mixture and became a hugely successful product, with annual sales exceeding US$6 billion in the year 2000.
Interestingly, the R and S forms of omeprazole have equivalent inhibitory activity against the H+/K+ ATPase. This is because omeprazole itself is not the chemical species directly responsible for inhibition of the proton pump. As illustrated below (Figure 1.1), omeprazole first undergoes an acid promoted rearrangement to afford a reactive sulphenamide intermediate (some steps are omitted in the reaction scheme below). The sulphenamide intermediate next reacts with a thiol (–SH) group on the ATPase, forming a covalent disulfide bond and thereby inhibiting the enzyme (Figure 1.1).
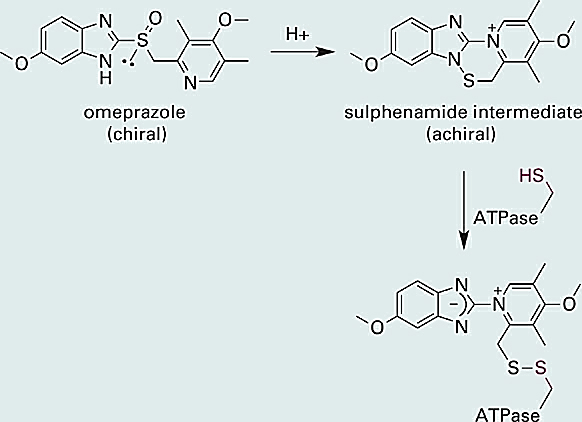
Figure 1.1 The chiral proton pump inhibitor omeprazole is converted in the parietal cells of the stomach into an achiral sulphenamide intermediate. The sulphenamide is electrophilic and reacts with a thiol (–SH) function on the proton pump (a H+/K+ ATPase) to form a disulfide bond, thereby inhibiting the pump and slowing the secretion of protons into the stomach.
You may have noted that the sulfur atom in the active sulphenamide intermediate is no longer attached to four different substituents and is therefore no longer a chirality center. Indeed, whereas omeprazole is chiral, the active sulphenamide intermediate is not, and this explains why the R and S forms of omeprazole have equivalent activity against the ATPase (both forms are converted to the same active intermediate). One might therefore expect little or no therapeutic benefit from a single-enantiomer form of omeprazole. However, in ~3% of Caucasians and 10–15% of Asians the R and S forms of omeprazole are metabolized differently in the liver. Subsequent clinical studies comparing the R and S forms of omeprazole with the racemic mixture showed that administration of (S)-omeprazole resulted in superior drug exposure in these “slow metabolizing” individuals. Thus, while the benefit is associated with a relatively small percentage of the population, it does constitute a therapeutic benefit and esomeprazole (marketed as Nexium®) received approval from the FDA in 2001.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|