


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-12-2015
Date: 1-12-2019
Date: 28-10-2020
|
The previous examples of aldol reactions and condensations used a common reactant as both the enolic donor and the electrophilic acceptor. The product in such cases is always a dimer of the reactant carbonyl compound. Aldol condensations between different carbonyl reactants are called crossed or mixed reactions, and under certain conditions such crossed aldol condensations can be effective.
Example 1.1: Mixed Aldol Reaction (One Product)
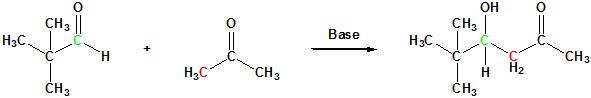
The success of these mixed aldol reactions is due to two factors. First, aldehydes are more reactive acceptor electrophiles than ketones, and formaldehyde is more reactive than other aldehydes. Second, aldehydes lacking alpha-hydrogens can only function as acceptor reactants, and this reduces the number of possible products by half. Mixed aldols in which both reactants can serve as donors and acceptors generally give complex mixtures of both dimeric (homo) aldols and crossed aldols. Because of this most mixed aldol reactions are usually not performed unless one reactant has no alpha hydrogens.
The following abbreviated formulas illustrate the possible products in such a case, red letters representing the acceptor component and blue the donor. If all the reactions occurred at the same rate, equal quantities of the four products would be obtained. Separation and purification of the components of such a mixture would be difficult.
ACH2CHO + BCH2CHO + NaOH  A–A + B–B + A–B + B–A
A–A + B–B + A–B + B–A
Example 1.2: Products of a Mixed Aldol Reaction
The aldol condensation of ketones with aryl aldehydes to form α,β-unsaturated derivatives is called the Claisen-Schmidt reaction.
Example 1.3: Claisen-Schmidt Reaction



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|