


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-7-2019
Date: 17-9-2019
Date: 14-7-2019
|
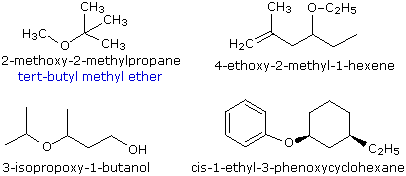
Ethers are compounds having two alkyl or aryl groups bonded to an oxygen atom, as in the formula R1–O–R2. The ether functional group does not have a characteristic IUPAC nomenclature suffix, so it is necessary to designate it as a substituent. To do so the common alkoxy substituents are given names derived from their alkyl component (Table 1.1):
| Alkyl Group | Name | Alkoxy Group | Name |
|---|---|---|---|
| CH3– | Methyl | CH3O– | Methoxy |
| CH3CH2– | Ethyl | CH3CH2O– | Ethoxy |
| (CH3)2CH– | Isopropyl | (CH3)2CHO– | Isopropoxy |
| (CH3)3C– | tert-Butyl | (CH3)3CO– | tert-Butoxy |
| C6H5– | Phenyl | C6H5O– | Phenoxy |
The smaller, shorter alkyl group becomes the alkoxy substituent. The larger, longer alkyl group side becomes the alkane base name. Each alkyl group on each side of the oxygen is numbered separately. The numbering priority is given to the carbon closest to the oxgen. The alkoxy side (shorter side) has an "-oxy" ending with its corresponding alkyl group. For example, CH3CH2CH2CH2CH2-O-CH2CH2CH3 is 1-propoxypentane. If there is cis or trans stereochemistry, the same rule still applies.
Example 1.1
Examples of ethers include CH3CH2OCH2CH3, diethyl ether (sometimes referred to as ether), and CH3OCH2CH2OCH3, ethylene glycol dimethyl ether (glyme).



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|