آخر المواضيع المضافة

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2019
Date: 21-10-2019
Date: 29-5-2017
|
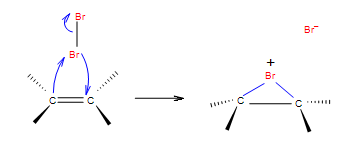
Before constructing the mechanism let us summarize conditions for this reaction. We will use Br2 in our example for halogenation of ethylene.
| Nucleophile | Double bond in alkene |
| Electrophile | Br2, Cl2 |
| Regiochemistry | not relevant |
|
Stereochemistry |
ANTI |
Step 1: In the first step of the addition the Br-Br bond polarizes, heterolytic cleavage occurs and Br with the positive charge forms a intermediate cycle with the double bond.
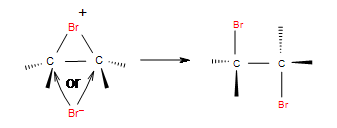
Step 2: In the second step, bromide anion attacks any carbon of the bridged bromonium ion from the back side of the cycle. Cycle opens up and two halogens are in the position anti.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
خدمات متعددة يقدمها قسم الشؤون الخدمية للزائرين
|
|
|