


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2018
Date: 25-9-2019
Date: 11-1-2020
|
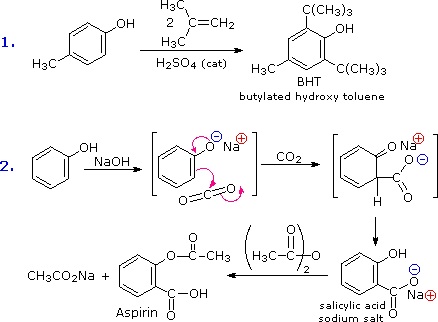
The facility with which the aromatic ring of phenols and phenol ethers undergoes electrophilic substitution has been noted. Two examples are shown in the following diagram. The first shows the Friedel-Crafts synthesis of the food preservative BHT from para-cresol. The second reaction is interesting in that it further demonstrates the delocalization of charge that occurs in the phenolate anion. Carbon dioxide is a weak electrophile and normally does not react with aromatic compounds; however, the negative charge concentration on the phenolate ring enables the carboxylation reaction shown in the second step. The sodium salt of salicylic acid is the major product, and the preference for ortho substitution may reflect the influence of the sodium cation. This is called the Kolbe-Schmidt reaction, and it has served in the preparation of aspirin, as the last step illustrates.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|