


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-1-2022
Date: 27-8-2018
Date: 27-8-2018
|
Even though the structures look the same, the formal charge (FC) may not be. Formal charges are charges that are assigned to a specific atom in a molecule. If computed correctly, the overall formal charge of the molecule should be the same as the oxidation charge of the molecule (the charge when you write out the empirical and molecular formula) We want to choose the resonance structure with the least formal charges that add up to zero or the charge of the overall molecule.
The equation for finding Formal Charge is:
Formal Charge = (number of valence electrons in free orbital) - (number of lone-pair electrons) - ( 12
number bond pair electrons)
The formal charge has to equal the molecule's overall charge.
Ex.) CNS- has an overall charge of -1, so the Lewis structure's formal charge has to equal -1.
See Lewis Structure for more information.
Example 1: Thiocyanate Ion Consider the thiocyanate (CNS-) ion.
SOLUTION
1. Find the Lewis Structure of the molecule. (Remember the Lewis Structure rules.)
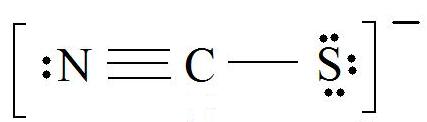
2. Resonance: All elements want an octet, and we can do that in multiple ways by moving the terminal atom's electrons around (bonds too).
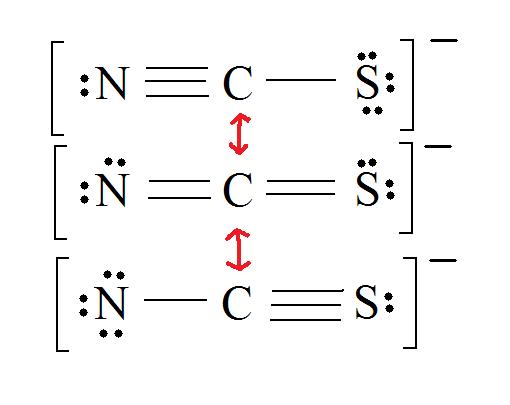
3. Assign Formal Charges
Formal Charge = (number of valence electrons in free orbital) - (number of lone-pair electrons) - ( 12
number bond pair electrons)
Remember to determine the number of valence electron each atom has before assigning Formal Charges
C = 4 valence e-, N = 5 valence e-, S = 6 valence e-, also add an extra electron for the (-1) charge. The total of valence electrons is 16.
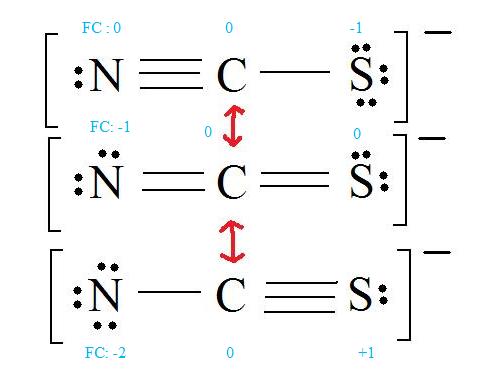
4. Find the most ideal resonance structure. (Note: It is the one with the least formal charges that adds up to zero or to the molecule's overall charge.)
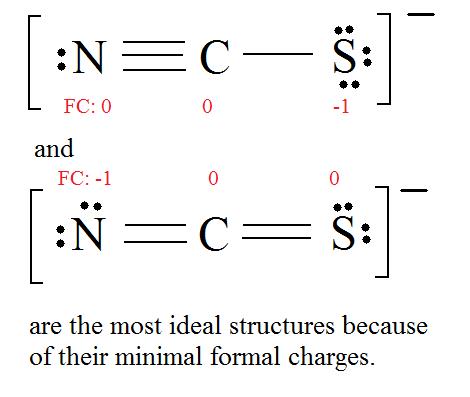
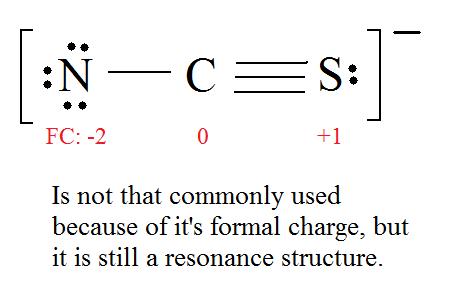
5. Now we have to look at electronegativity for the "Correct" Lewis structure.
The most electronegative atom usually has the negative formal charge, while the least electronegative atom usually has the positive formal charges.
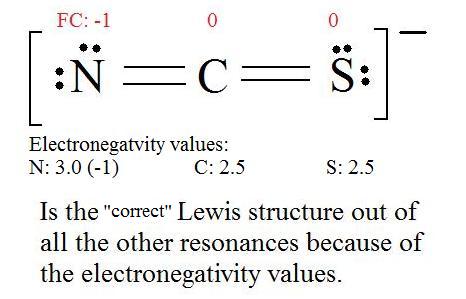



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|