

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-6-2016
Date: 24-7-2019
Date: 4-9-2019
|
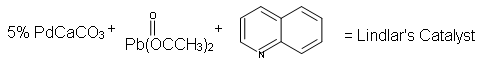
Like alkenes, alkynes readily undergo catalytic hydrogenation, either to cis or trans alkenes, or to alkanes, depending on the reaction employed.
The catalytic addition of hydrogen to 2-butyne provides heat of reaction data that reflect the relative thermodynamic stabilities of these hydrocarbons, as shown in the diagram to the right. From the heats of hydrogenation, shown in blue in units of kcal/mole, it would appear that alkynes are thermodynamically less stable than alkenes to a greater degree than alkenes are less stable than alkanes. The standard bond energies for carbon-carbon bonds confirm this conclusion. Thus, a double bond is stronger than a single bond, but not twice as strong. The difference ( 63 kcal/mole ) may be regarded as the strength of the π-bond component. Similarly, a triple bond is stronger than a double bond, but not 50% stronger. Here the difference ( 54 kcal/mole ) may be taken as the strength of the second π-bond. The 9 kcal/mole weakening of this second π-bond is reflected in the heat of hydrogenation numbers ( 36.7 - 28.3 = 8.4 ).
Since alkynes are thermodynamically less stable than alkenes, we might expect addition reactions of the former to be more exothermic and relatively faster than equivalent reactions of the latter. In the case of catalytic hydrogenation, the usual Pt and Pd hydrogenation catalysts are so effective in promoting addition of hydrogen to both double and triple carbon-carbon bonds that the alkene intermediate formed by hydrogen addition to an alkyne cannot be isolated. A less efficient catalyst, Lindlar's catalyst, prepared by deactivating (or poisoning) a conventional palladium catalyst by treating it with lead acetate and quinoline, permits alkynes to be converted to alkenes without further reduction to an alkane.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|