


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-5-2016
Date: 5-9-2017
Date: 28-8-2017
|
Petroleum is converted to useful products such as gasoline in three steps: distillation, cracking, and reforming. Recall from Chapter 1 "Introduction to Chemistry" that distillation separates compounds on the basis of their relative volatility, which is usually inversely proportional to their boiling points. Part (a) in Figure 3.8.1 shows a cutaway drawing of a column used in the petroleum industry for separating the components of crude oil. The petroleum is heated to approximately 400°C (750°F), at which temperature it has become a mixture of liquid and vapor. This mixture, called the feedstock, is introduced into the refining tower. The most volatile components (those with the lowest boiling points) condense at the top of the column where it is cooler, while the less volatile components condense nearer the bottom. Some materials are so nonvolatile that they collect at the bottom without evaporating at all. Thus the composition of the liquid condensing at each level is different. These different fractions, each of which usually consists of a mixture of compounds with similar numbers of carbon atoms, are drawn off separately. Part (b) in Figure 3.8.1 shows the typical fractions collected at refineries, the number of carbon atoms they contain, their boiling points, and their ultimate uses. These products range from gases used in natural and bottled gas to liquids used in fuels and lubricants to gummy solids used as tar on roads and roofs.
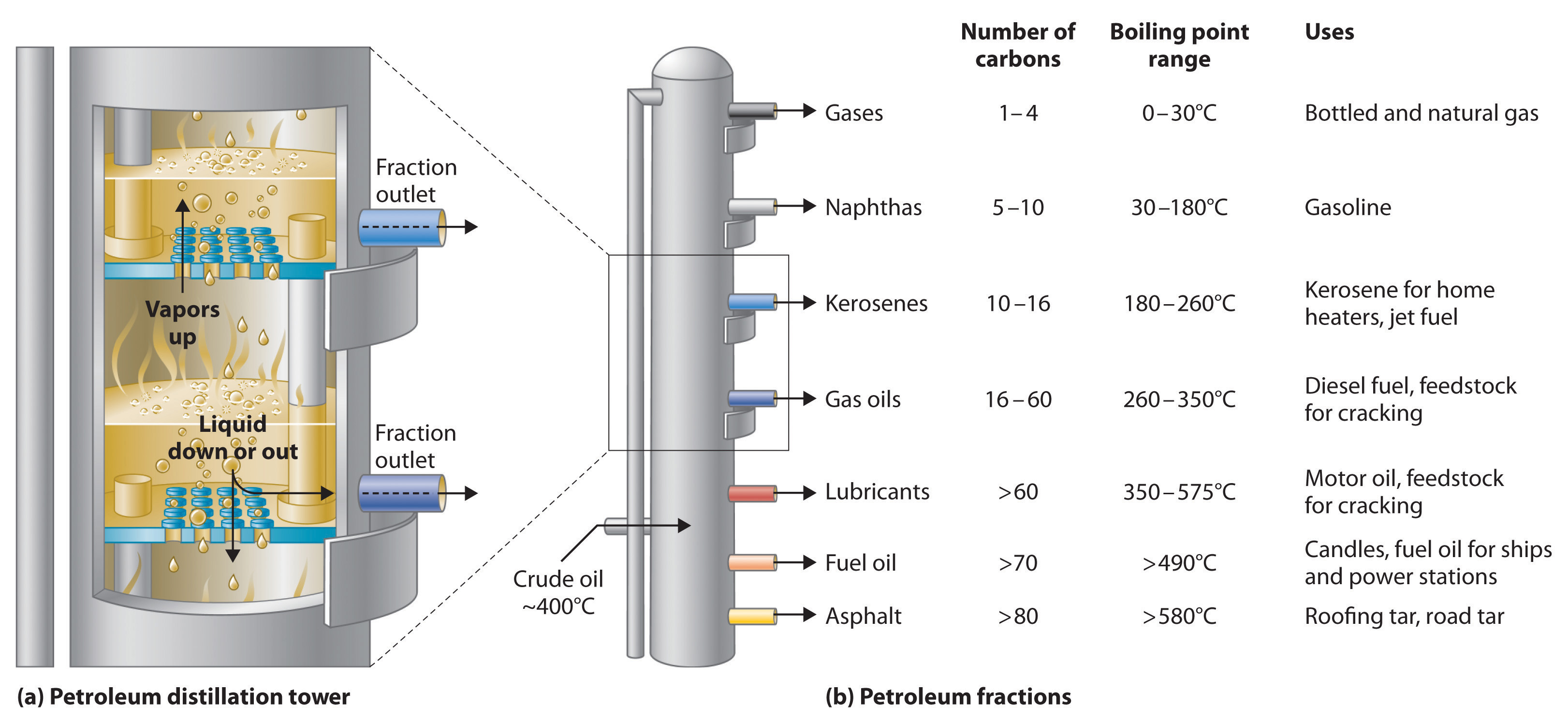
Figure 3.8.1: The Distillation of Petroleum. (a) This is a diagram of a distillation column used for separating petroleum fractions. (b) Petroleum fractions condense at different temperatures, depending on the number of carbon atoms in the molecules, and are drawn off from the column. The most volatile components (those with the lowest boiling points) condense at the top of the column, and the least volatile (those with the highest boiling points) condense at the bottom.
The economics of petroleum refining are complex. For example, the market demand for kerosene and lubricants is much lower than the demand for gasoline, yet all three fractions are obtained from the distillation column in comparable amounts. Furthermore, most gasolines and jet fuels are blends with very carefully controlled compositions that cannot vary as their original feedstocks did. To make petroleum refining more profitable, the less volatile, lower-value fractions must be converted to more volatile, higher-value mixtures that have carefully controlled formulas. The first process used to accomplish this transformation is cracking, in which the larger and heavier hydrocarbons in the kerosene and higher-boiling-point fractions are heated to temperatures as high as 900°C. High-temperature reactions cause the carbon–carbon bonds to break, which converts the compounds to lighter molecules similar to those in the gasoline fraction. Thus in cracking, a straight-chain alkane with a number of carbon atoms corresponding to the kerosene fraction is converted to a mixture of hydrocarbons with a number of carbon atoms corresponding to the lighter gasoline fraction. The second process used to increase the amount of valuable products is called reforming; it is the chemical conversion of straight-chain alkanes to either branched-chain alkanes or mixtures of aromatic hydrocarbons. Using metals such as platinum brings about the necessary chemical reactions. The mixtures of products obtained from cracking and reforming are separated by fractional distillation.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|