
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 15-1-2017
Date: 28-9-2018
Date: 22-9-2018
|
Molecularity of an Elementary Reaction
The molecularity of an elementary reaction is defined as : the number of reactant molecules involved in a reaction.
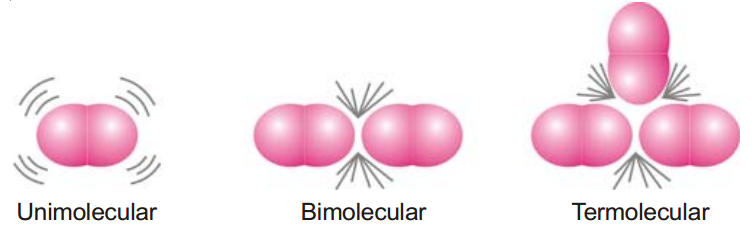
Thus the molecularity of an elementary reaction is 1, 2, 3, etc., according as one, two or three reactant molecules are participating in the reaction. The elementary reactions having molecularity 1, 2 and 3 are called unimolecular, bimolecular and termolecular respectively. Thus we have :
(a) Unimolecular reactions : (molecularity = 1)
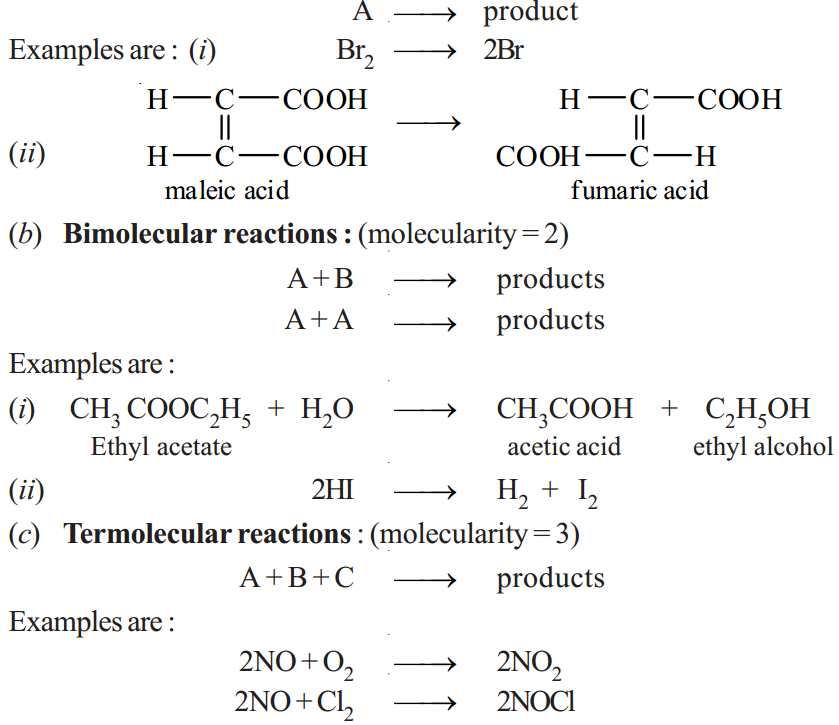



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|