


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-7-2019
Date: 8-7-2019
Date: 12-9-2019
|
Exhaustive base-catalyzed halogenation of methyl ketones is accompanied by C–C bond cleavage, yielding a carboxylate salt and haloform ( X3CH ). As shown in the diagram, an enolate conjugate base reacts with elemental halogen to form an α-halo ketone. This process is repeated until the α-site is fully halogenated. The trihalo derivatives formed from methyl ketones are then cleaved by base attack at the carbonyl group, reflecting the relative stability of the trihalomethyl anion as a leaving group.

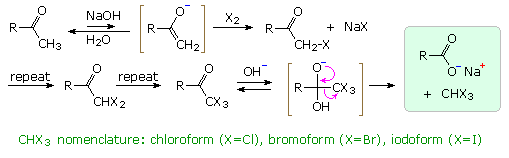
Before nmr became an essential tool for structure elucidation, a variant of the haloform reaction called the iodoform test was used to identify methyl ketones, as well as alcohols that gave methyl ketones when oxidized. By clicking on the diagram an illustration of this test will appear as reaction 1. The iodoform product is an easily identifiable, bright yellow, water-insoluble solid with a characteristic odor.
The haloform reaction has also been used to prepare carboxylic acids, as in the synthesis of trimethylacetic acid from acetone shown in reaction 2. Dissolving metal reduction of acetone gives the diol pinacol, which then undergoes acid-catalyzed rearrangement to the methyl ketone pinacolone.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|