


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-12-2019
Date: 16-12-2019
Date: 13-12-2019
|
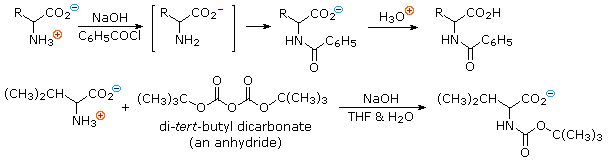
Reactions of α-Amino Acids : Amine Acylation
In order to convert the amine function of an amino acid into an amide, the pH of the solution must be raised to 10 or higher so that free amine nucleophiles are present in the reaction system. Carboxylic acids are all converted to carboxylate anions at such a high pH, and do not interfere with amine acylation reactions. The following two reactions are illustrative. In the first, an acid chloride serves as the acylating reagent. This is a good example of the superior nucleophilicity of nitrogen in acylation reactions, since water and hydroxide anion are also present as competing nucleophiles. A similar selectivity favoring amines was observed in the Hinsberg test. The second reaction employs an anhydride-like reagent for the acylation. This is a particularly useful procedure in peptide synthesis, thanks to the ease with which the t-butylcarbonyl (t-BOC) group can be removed at a later stage. Since amides are only weakly basic ( pKa~ -1), the resulting amino acid derivatives do not display zwitterionic character, and may be converted to a variety of carboxylic acid derivatives.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|