

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Thermal analysis
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص247-249
2025-09-02
58
Thermal analysis
Key points: Thermal methods include thermogravimetric analysis, differential thermal analysis, and differential scanning calorimetry. Thermal analysis is the analysis of a change in a property of a sample induced by heating. The sample is usually a solid and the changes that occur include melting, phase transition, sublimation, and decomposition. The analysis of the change in the mass of a sample on heating is known as thermo gravimetric analysis (TGA). The measurements are carried out using a thermobalance, which consists of an electronic microbalance, a temperature-programmable furnace, and a controller, which enables the sample to be simultaneously heated and weighed (Fig. 8.42). The sample is weighed into a sample holder and then suspended from the balance within the furnace. The temperature of the furnace is usually increased linearly, but more com plex heating schemes, isothermal heating (heating that maintains constant temperature at a phase transition), and cooling protocols can also be used. The balance and furnace are situated within an enclosed system so that the atmosphere can be controlled. That atmosphere may be inert or reactive, depending on the nature of the investigation, and can be static or flowing. A flowing atmosphere has the advantage of carrying away any volatile or corrosive species and prevents the condensation of reaction products. In addition, any species produced can be fed into a mass spectrometer for identification. Thermogravimetric analysis is most useful for desorption, decomposition, dehydration, and oxidation processes. For example, the thermogravimetric curve for CuSO4.5H2O from room temperature to 300ºC shows three stepwise mass losses (Fig. 8.43), corresponding to the three stages in the dehydration to form first CuSO4 .3H2O, then CuSO4.H2O, and finally CuSO4.
The most widely used thermal method of analysis is differential thermal analysis (DTA). In this technique the temperature of the sample is compared to that of a reference material while they are both subjected to the same heating procedure. In a DTA instrument, the sample and reference are placed in low thermal conductivity sample holders that are then located within cavities in a block in the furnace. Common reference samples for the analysis of inorganic compounds are alumina, Al2O3, and carborundum, SiC. The temperature of the furnace is increased linearly and the difference in temperature between the sample and the reference is plotted against the furnace temperature. If an endothermic event takes place within the sample, the temperature of the sample lags behind that of the reference and a minimum is observed on the DTA curve. If an exothermal event takes place, the temperature of the sample rises above that of the reference and a maximum is observed on the curve. The area under the endotherm or exotherm (the resulting curve in each case) is related to the enthalpy change accompanying the thermal event. A technique closely related to DTA is differential scanning calorimetry (DSC). In DSC, the sample and the reference are maintained at the same temperature throughout the heat ing procedure by using separate power supplies to the sample and reference holders. Any difference between the power supplied to the sample and reference is recorded against the furnace temperature. Thermal events appear as deviations from the DSC baseline as either endotherms or exotherms, depending on whether more or less power has to be supplied to the sample relative to the reference. In DSC, endothermic reactions are usually represented as positive deviations from the baseline, corresponding to increased power supplied to the sample. Exothermic events are represented as negative deviations from the baseline. The information obtained from DTA and DSC is very similar. The former can be used up to higher temperatures although the quantitative data, such as the enthalpy of a phase change, obtained from DSC are more reliable. Both DTA and DSC are used for ‘finger print’ comparison of the results obtained from a sample with those of a reference material. Information about the temperatures and enthalpy changes of transitions, such as a change in structure or melting, can be extracted.
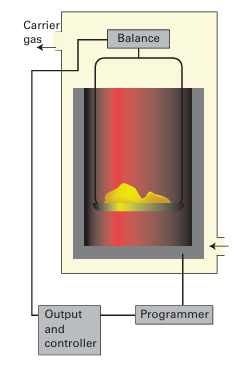
Figure 8.42 A thermogravimetric analyser: the mass of the sample is monitored as the temperature is raised.
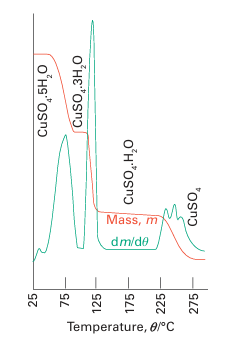
Figure 8.43 The thermogravimetric curve obtained for CuSO4 .5H2O as the temperature is raised from 20°C to 500°C. The red line is the mass of the sample and the green line is its first derivative (the slope of the red line).

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















