

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Photoelectron spectroscopy
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص241-242
2025-09-02
63
Photoelectron spectroscopy
Key point: Photoelectron spectroscopy is used to determine the energies and order of orbitals in mol ecules and solids by analysing the kinetic energies of photoejected electrons. The basis of photoelectron spectroscopy (PES) is the measurement of the kinetic energies of electrons (photoelectrons) emitted by ionization of a sample that is irradiated with high energy monochromatic radiation (Fig. 8.31). It follows from the conservation of energy that the kinetic energy of the ejected photoelectrons, Ek, is related to their ionization energies, Ei, from their orbitals by the relation
Ek=hv – Ei
where is the frequency of the incident radiation. Koopmans’ theorem states that the ionization energy is equal to the negative of the orbital energy, so the determination of the kinetic energies of photoelectrons can be used to determine orbital energies. The theorem assumes that the energy involved in electron reorganization after ionization is offset by the increase in electron–electron repulsion energy as the orbital contracts. This approximation is usually taken as reasonably valid.
There are two major types of photoionization technique, X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS). Although much more intense sources can be obtained using synchrotron beam lines, the standard laboratory source for XPS is usually a magnesium or aluminium anode that is bombarded by a high energy electron beam. This bombardment results in radiation at 1.254 and 1.486 keV, respectively, due to the transition of a 2p electron into a vacancy in the 1s orbital caused by ejection of an electron. These energetic photons cause ionizations from core orbitals in other elements that are present in the sample; the ionization energies are characteristic of the element and its oxidation state. Because the linewidth is high (usually 1–2 eV), XPS is not suitable for probing fine details of valence orbitals but can be used to study the band structures of solids. The mean free path of electrons in a solid is only about 1 nm, so XPS is suitable for surface elemental analysis, and in this application it is commonly known as electron spectroscopy for chemical analysis (ESCA).
The source for UPS is typically a helium discharge lamp that emits He(I) radiation (21.22 eV) or He(II) radiation (40.8 eV). Linewidths are much smaller than in XPS, so the resolution is far greater. The technique is used to study valence-shell energy levels and the vibrational fine structure often provides important information on the bonding or antibonding character of the orbitals from which electrons are ejected (Fig. 8.32). When the electron is removed from a nonbonding orbital the product is formed in its vibrational ground state, and a narrow line is observed. However, when the electron is removed from a bonding or antibonding orbital the resulting ion is formed in several different vibrational states and extensive fine structure is observed. Bonding and antibonding orbitals can be distinguished by determining whether the vibrational frequencies in the resulting ion are higher or lower than for the original molecule. Another useful aid is the comparison of photoelectron intensities for a sample irradiated with He(I) and He(II). The higher energy source preferentially ejects electrons from d or f orbitals, allowing these contributions to be distinguished from s and p orbitals, for which He(I) causes higher intensities. The origin of this effect lies in differences in absorption cross-sections (see Further reading).
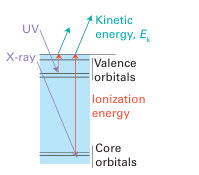
Figure 8.31 In photoelectron spectroscopy, high-energy electromagnetic radiation (UV for the ejection of valence electrons, X-ray for core electrons) expels an electron from its orbital, and the kinetic energy of the photoelectron is equal to the difference between the photon energy and the ionization energy of the electron.
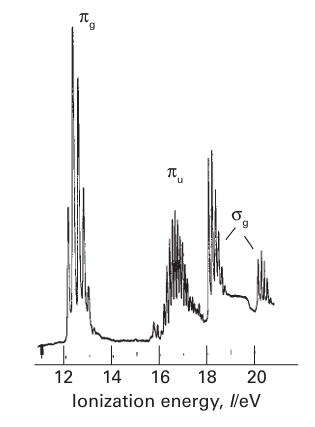
Figure 8.32 The UV photoelectron spectra of O2. Loss of an electron from 2Ϭg (see the MO energy-level diagram, Fig. 2.12) gives rise to two bands because the unpaired electron that remains can be parallel or antiparallel to the two unpaired electrons in the 1πg orbitals.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















