

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
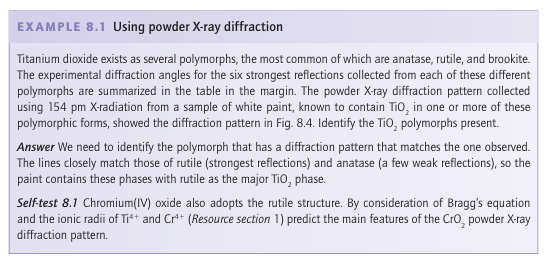
Powder X-ray diffraction
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص224-225
2025-09-01
94
Powder X-ray diffraction
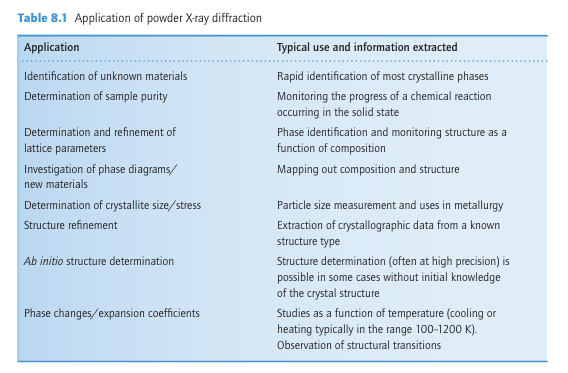
Key point: Powder X-ray diffraction is used mainly for phase identification and the determination of lattice parameters and lattice type. A powdered (polycrystalline) sample contains an enormous number of very small crystallites, typically 0.1 to 10μm in dimension and orientated at random. An X-ray beam striking a polycrystalline sample is scattered in all directions; at some angles, those given by Bragg’s equation, constructive interference occurs. As a result, each set of planes of atoms with lattice spacing d gives rise to a cone of diffraction intensity. Each cone consists of a set of closely spaced diffracted rays, each one of which represents diffraction from a single crystallite within the powder sample (Fig. 8.2). With a very large number of crystallites these rays merge together to form the diffraction cone. A powder diffractometer (Fig. 8.3a) uses an electronic detector to measure the angles of the diffracted beams. Scanning the detector around the sample along the circumference of a circle cuts through the diffraction cones at the various diffraction maxima and the intensity of the X-rays detected is recorded as a function of the detector angle (Fig. 8.3b). The number and positions of the reflections depend on the cell parameters, crystal system, lattice type, and wavelength used to collect the data; the peak intensities depend on the types of atoms present and their positions. Nearly all crystalline solids have a unique powder X-ray diffraction pattern in terms of the angles of the reflections and their intensities. In mixtures of compounds, each crystalline phase present contributes to the powder diffraction pattern its own unique set of reflection angles and intensities. Typically, the method is sensitive enough to detect a small level (5 to 10 per cent by mass) of a particular crystalline component in a mixture. The effectiveness of powder X-ray diffraction has led to it becoming the major technique for the characterization of polycrystalline inorganic materials (Table 8.1). Many of the powder diffraction data sets collected from inorganic, organometallic, and organic com pounds have been compiled into a database by the Joint Committee on Powder Diffraction Standards (JCPDS). This database, which contains over 50 000 unique powder X-ray diffraction patterns, can be used like a fingerprint library to identify an unknown material from its powder pattern alone. Powder X-ray diffraction is used routinely in the investigation of phase formation and changes in structures of solids. The synthesis of a metal oxide can be verified by collecting a powder diffraction pattern and demonstrating that the data are consistent with a single pure phase of that material. Indeed, the progress of a chemical reaction is often monitored by observing the formation of the product phase at the expense of the reactants. Basic crystallographic information, such as lattice parameters, can normally be extracted easily from powder X-ray diffraction data, usually with high precision. The presence or absence of certain reflections in the diffraction pattern permits the determination of the lattice type. In recent years the technique of fitting the intensities of the peaks in the dif fraction pattern has become a popular method of extracting structural information such as atomic positions. The analysis, which is known as the Rietveld method, involves fitting a calculated diffraction pattern to the experimental trace. The technique is not as powerful as the single-crystal methods, for it gives less accurate atomic positions, but has the advantage of not requiring the growth of a single crystal.
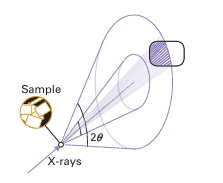
Figure 8.2 A cone of diffraction that results from X-ray scattering by a powdered sample. The cone consists of thousands of individual diffraction spots from individual crystallites that merge together.
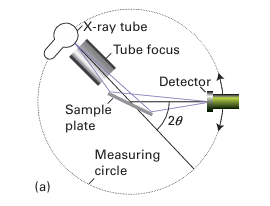
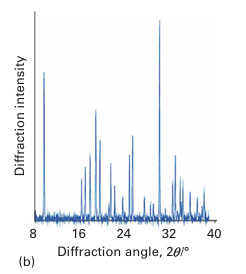
Figure 8.3 (a) Schematic diagram of a powder diffractometer operating in reflection mode in which the X-ray scattering occurs from a sample mounted as a flat plate. For weakly absorbing compounds the samples may be mounted in a capillary and the diffraction data collected in transmission mode. (b) The form of a typical powder diffraction pattern showing a series of reflections as a function of angle.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















