


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-2-2018
Date: 5-7-2020
Date: 25-6-2019
|
Nitrogen halides
Nitrogen is restricted to an octet of valence electrons and does not form pentahalides. The fact that nitrogen pentahalides are not known has also been attributed to the steric crowding of five halogen atoms around the small N atom.
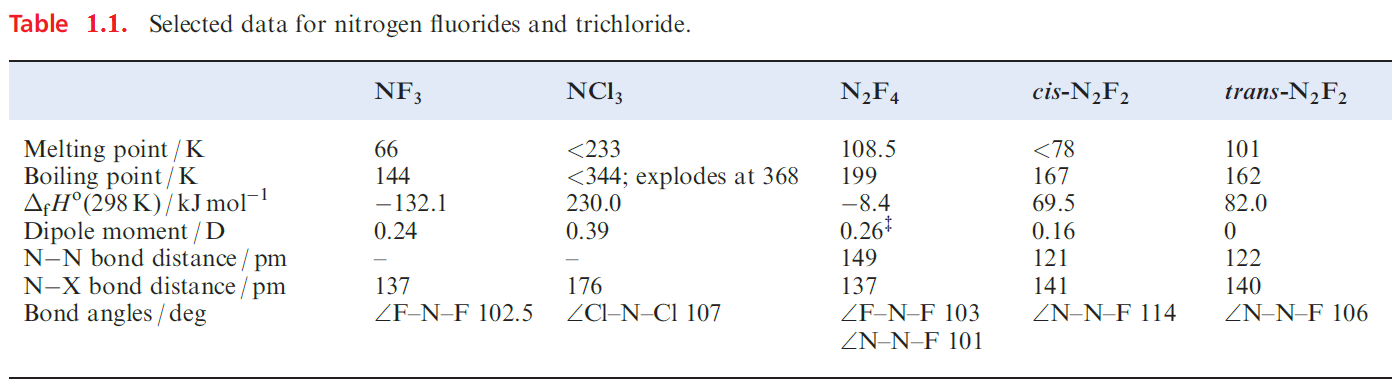
Important nitrogen halides are NX3 (X= F, Cl), N2F4 and N2F2, selected properties for which are listed in Table 1.1; NBr3 and NI3 exist but are less well characterized than NF3 and NCl3.
Nitrogen trifluoride which must be carried out in a controlled manner, or by electrolysis of anhydrous NH4F/HF mixtures.

NF3 is the most stable of the trihalides of nitrogen, being the only one to have a negative value of ΔfHo (Table 1.1). It is a colourless gas which is resistant to attack by acids and alkalis, but is decomposed by sparking with H2 . The resistance towards hydrolysis parallels that observed for the carbon tetrahalides.

The gas-phase structure of NF3 is trigonal pyramidal and the molecular dipole moment is very small (Table 1.1). In contrast to NH3 and PF3, NF3 shows no donor properties.
Nitrogen trichloride is an oily, yellow liquid at 289 K, but it is highly endothermic and dangerously explosive (Table1.1). The difference in stabilities of NF3 and NCl3 lies in the relative bond strengths of N_F over N_Cl, and of Cl2 over F2. .
Diluted with air, NCl3 is used for bleaching flour since hydrolysis by moisture forms HOCl. Alkalis hydrolyse NCl3 according to equation 1.16.
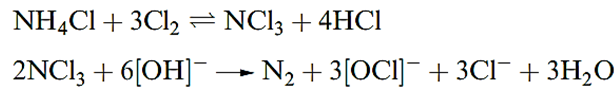
Nitrogen tribromide is more reactive than NCl3, and explodes at temperatures as low as 175 K.

Nitrogen triiodide has been made by reacting IF with boron nitride in CFCl3. Although NI3 is stable at 77K and has been characterized by IR, Raman and 15N NMR spectroscopies, it is highly explosive at higher temperatures (ΔfHo (NI3,g) = 287 kJ mol-1). The reaction between concentrated aqueous NH3 and [I3]- yields NH3 :NI3, black crystals of which are dangerously explosive (ΔfHo(NH3:NI3,s) = 146 kJ mol-1) as the compound decomposes to NH3, N2 and I2.
The nitrogen fluorides N2F4 and N2F2 can be obtained from reactions 1.18 and 1.19; properties of these fluorides are listed in Table 1.1, and both fluorides are explosive.
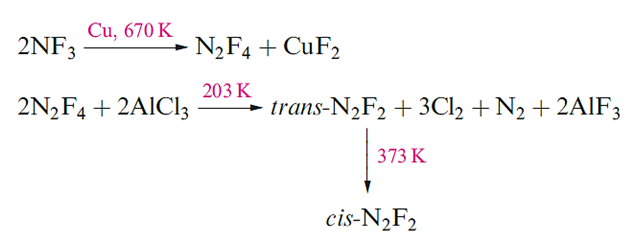
The structure of N2F4 resembles that of hydrazine, except that both the gauche and trans (staggered) conformers are present in the liquid and gas phases. At temperatures above 298 K, N2F4 reversibly dissociates into blue NF2. radicals which undergo many interesting reactions.
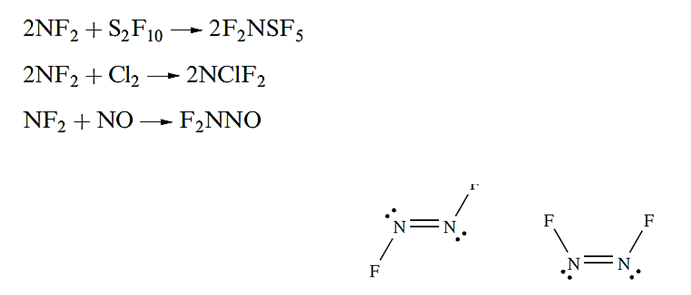
Dinitrogen difluoride, N2F2, exists in both the trans- and cis-forms (above), with the cis-isomer being thermodynamically the more stable of the two but also the more reactive. Reaction above gives a selective method of preparing trans-N2F2; isomerization by heating gives a mixture of isomers from which cis-N2F2 can be isolated by treatment with AsF5 . Mixture of isomers:

Reaction 14.63 illustrates the ability of N2F2 to donate F- to strong acceptors such as AsF5 and SbF5, a reaction type shared by N2F4 . The cation [NF4]+ is formed in reaction below. We return to the properties of AsF5 and SbF5 later.
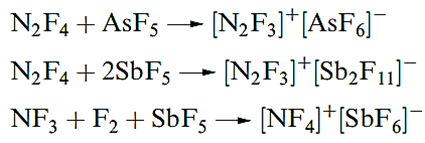



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|