


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-10-2018
Date: 16-12-2018
Date: 24-10-2018
|
Physical properties of group 1 metals
General properties
The alkali metals illustrate, more clearly than any other group of elements, the influence of increase in atomic and ionic size on physical and chemical properties. Thus, the group 1 metals are often chosen to illustrate general principles. Some physical properties of the group 1 metals are given in Table 1.1.

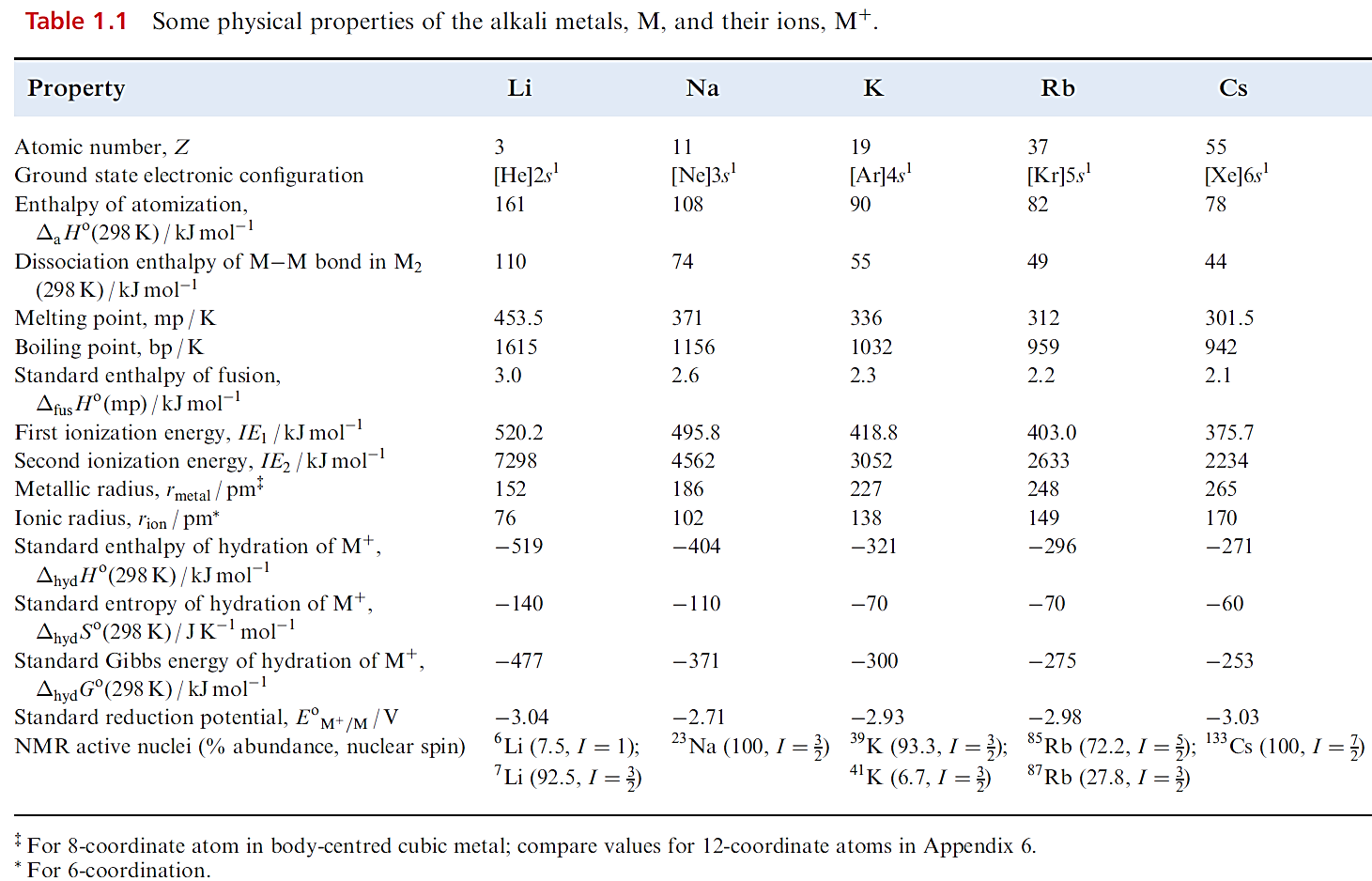
and down group 1, differences in these energy changes almost cancel out, resulting in similar EoM+/M values. The lower reactivity of Li towards H2O is kinetic rather than thermodynamic in origin; Li is a harder and higher melting metal, is less rapidly dispersed, and reacts more slowly than its heavier congeners. In general, the chemistry of the group 1 metals is dominated by compounds containing M ions.
Considerations of lattice energies calculated using an electrostatic model provide a satisfactory understanding for the fact that ionic compounds are central to the chemistry of Na, K, Rb and Cs. That Li shows a so-called ‘anomalous’ behaviour and exhibits a diagonal relationship to Mg can be explained in terms of similar energetic considerations.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|