


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-4-2016
Date: 18-9-2017
Date: 14-8-2017
|
phthalic anhydride
Currently, phthalic anhydride is mainly produced through catalyzed oxidation of o-xylene. A variety of metal oxides are used as catalysts. A typical one is V2O5 + TiO2/Sb2O3. Approximate conditions for the vapor-phase oxidation are 375–435°C and 0.7 atmosphere. The yield of phthalic anhydride is about 85%:
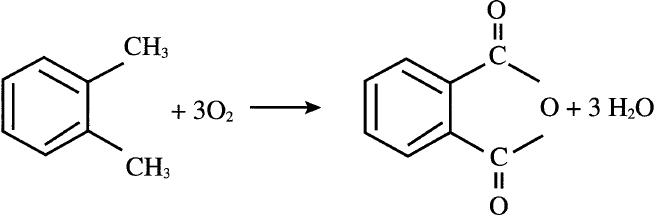
Liquid-phase oxidation of o-xylene also works at approximately 150°C. Cobalt or manganese acetate in acetic acid medium serves as a catalyst.The major by-products of this process are maleic anhydride, benzoic acid, and citraconic anhydride (methylmaleic anhydride). Maleic anhydride could be recovered economically. Phthalic anhydride’s main use is for producing plasticizers by reactions with C4–C10 alcohols. The most important polyvinyl chloride plasticizer is formed by the reaction of 2-ethylhexanol and phthalic anhydride:

Phthalic anhydride is also used to make polyester and alkyd resins. It is a precursor for phthalonitrile by an ammoxidation route used to produce phthalamide and phathilimide. The reaction scheme for producing phthalonitrile, phthalamide, and phathilimide is shown in Figure 1.1.
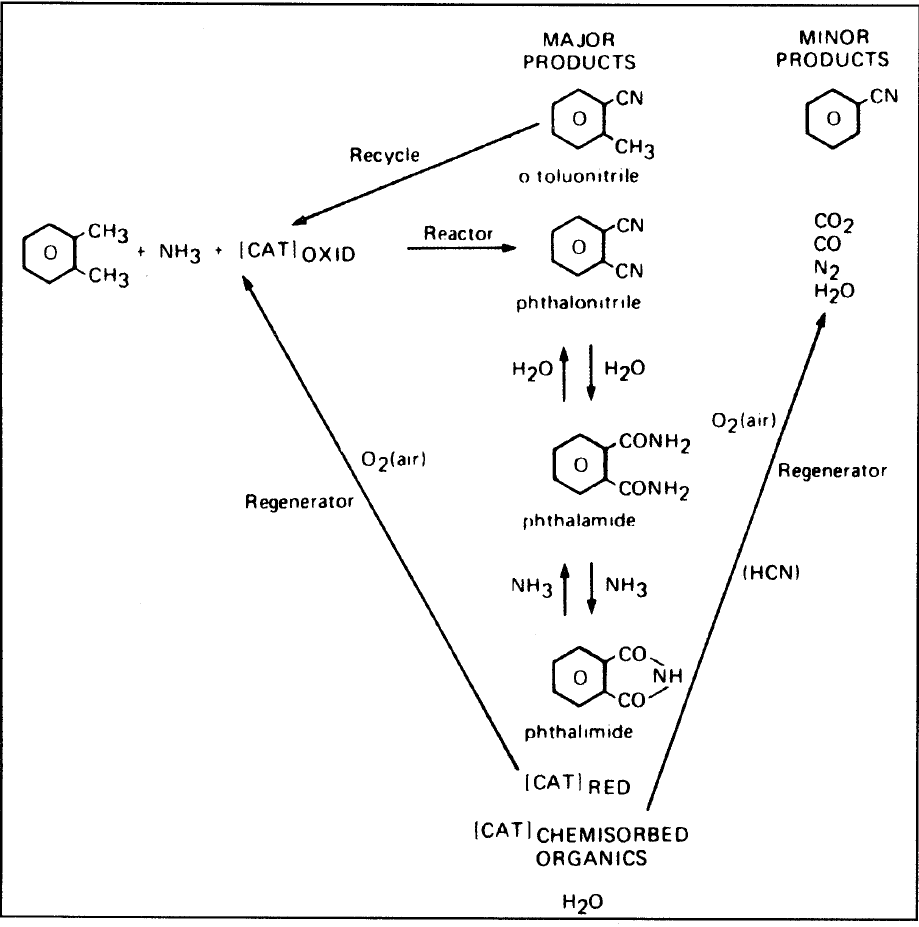
Figure 1.1. The reaction scheme for o-xylene to phthalonitrile.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|