
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-9-2017
Date: 13-8-2017
Date: 21-8-2017
|
Phenol from Benzoic Acid
The action of a copper salt converts benzoic acid to phenol. The copper, reoxidized by air, functions as a real catalyst. The Lummus process operates in the vapor phase at approximately 250°C. Phenol yield of 90% is possible:
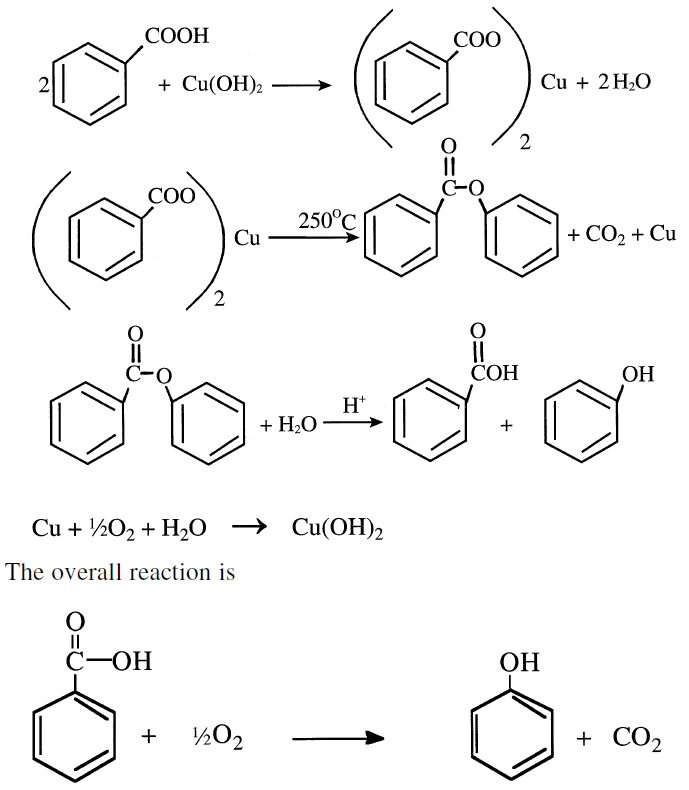
In the Lummus process (Figure 1.1), the reaction occurs in the liquid phase at approximately 220–240°C over Mg2+ + Cu2+ benzoate.
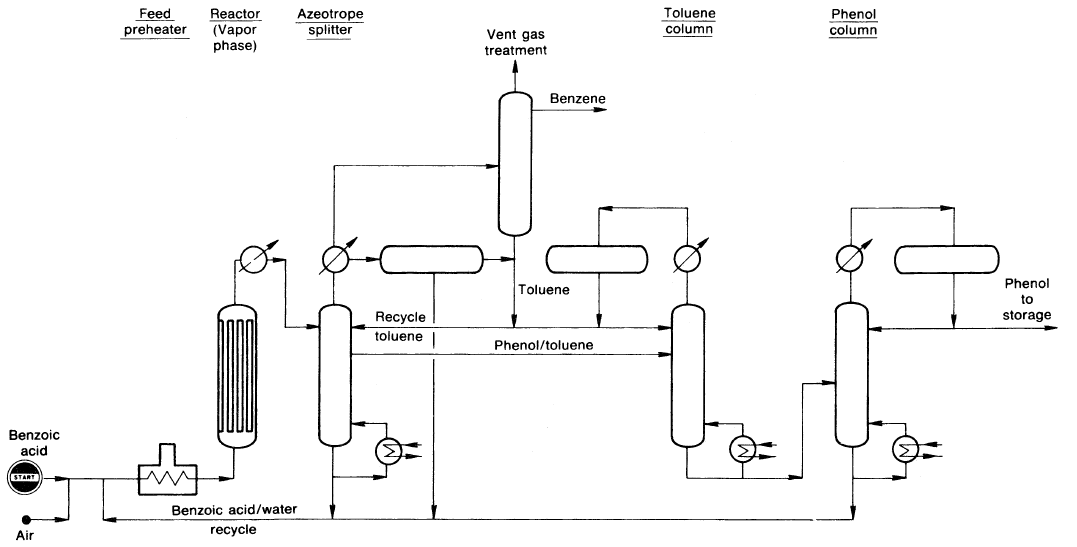
Figure 1.1. The Lummus benzoic-acid-to-phenol process.
Magnesium benzoate is an initiator, with the Cu2+ reduced to Cu1+. The copper (1) ions are reoxidized to copper (II) ions.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|