
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-12-2020
Date: 1-7-2017
Date: 2-7-2017
|
Effect of Changes in Temperature
When the temperature of a system at equilibrium is increased, a reaction occurs in the direction that absorbs heat. The above equilibrium is exothermic when the reaction proceeds to the right, ΔH° = -90 kJ. Thus if the temperature is increased, we expect the equilibrium to shift such as to absorb heat. Shifting to the right would liberate more heat, thus we expect a shift toward CO and H2. This conclusion can also be based on Eq. (1.1.). Since ΔH° is negative, an increase in temperature will decrease K, i. e., shift the equilibrium to the left
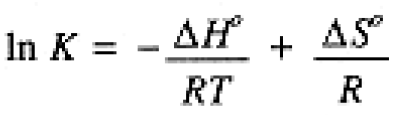 (1.1)
(1.1)



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|