


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-10-2018
Date: 10-3-2019
Date: 30-12-2018
|
Silicon
Silicon tetraalkyl and tetraaryl derivatives (R4Si), as well as alkyl or aryl silicon halides (RnSiCl4 - n, n = 1–3) can be prepared by reaction types 1.1–1.5. Note that variation in stoichiometry provides flexibility in synthesis, although the product specificity may be influenced by steric requirements of the organic substituents. Reaction 1.1 is used industrially (the Rochow process).
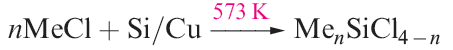 (1.1)
(1.1)
 (1.2)
(1.2)
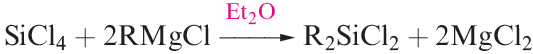 (1.3)
(1.3)
 (1.4)
(1.4)
The structures of these compounds are all similar: monomeric, with tetrahedrally sited Si and resembling their C analogues. Silicon_carbon bonds are relatively strong (the bond enthalpy term is 318 kJ mol-1) and R4Si derivatives possess high thermal stabilities. The stability of the Si_C bond is further illustrated by the fact that chlorination of Et4Si give ) ClCH2CH2)4Si, in contrast to the chlorination of R4Ge or R4Sn which yields RnGeCl4 - n or RnSnCl4- n (see equation 18.49). An important reaction of MenSiCl4_n (n = 1–3) is hydrolysis to produce silicones (e.g. equation 1.6).
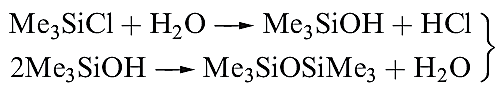 (1.5)
(1.5)
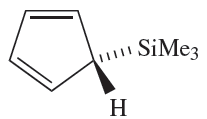
(1.1)
The reaction of Me3SiCl with NaCp leads to 1.1, in which the cyclopentadienyl group is η1. Related η1- complexes include) η1-C5Me5(2SiBr2 which reacts with anthracene/potassium to give the diamagnetic silylene(η5- C5Me5)2Si. In the solid state, two independent molecules are present (Figure 1.1a, b) which differ in the relative orientations of the cyclopentadienyl rings. In one molecule, the two C5-rings are parallel and staggered (compare Cp2Mg) whereas in the other, they are tilted (Figure 1.1c). We return to this observation at the end of Section 18.5. The reactions between R2SiCl2 and alkali metals or alkali metal naphthalides give cyclo-(R2Si)n by loss of Cl- and Si_Si bond formation. Bulky R groups favour small rings (e.g.(2,6-Me2C6H3)6Si3 and tBu6Si3) while smaller R substituents encourage the formation of large rings (e.g. Me12Si6;Me14Si7 and Me32Si16).
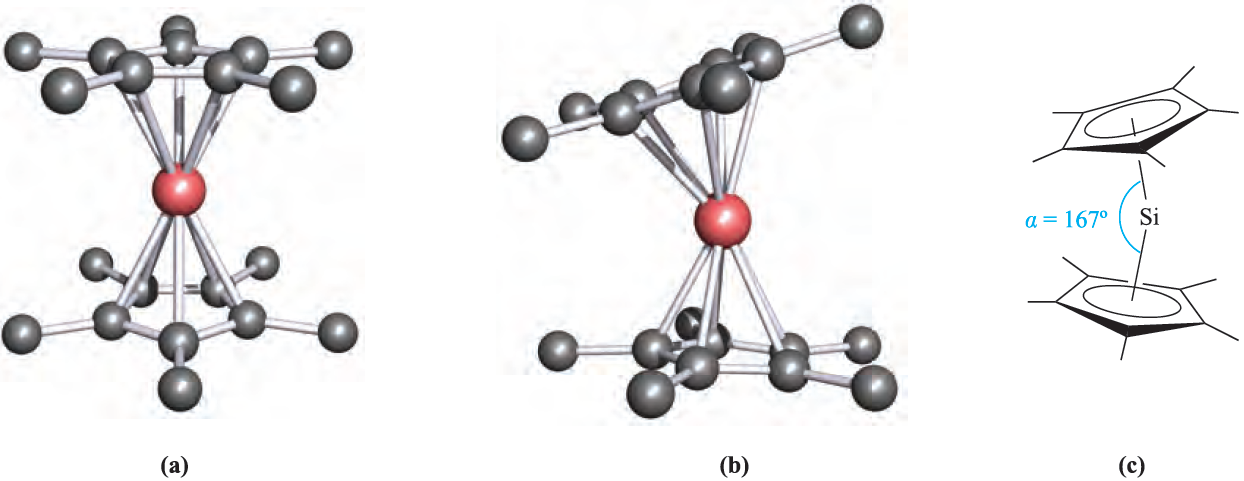
Fig. 1.1 The solid state structure of (η5-C5Me5(2Si contains two independent molecules. (a) In the first molecule, the cyclopentadienyl rings are co-parallel, while (b) in the other molecule they are mutually tilted; (c) the tilt angle is measured as angle α [P. Jutzi et al. (1986) Angew. Chem. Int. Ed. Engl., vol. 25, p. 164]. Hydrogen atoms are omitted for clarity; colour code: Si, pink; C, grey.
Reaction 1.6 is designed to provide a specific route to a particular ring size.
 (1.6)
(1.6)
Silylenes, R2Si (analogues of carbenes), can be formed by a variety of methods, for example, the photolysis of cyclic or linear organopolysilanes. As expected, R2Si species are highly reactive, undergoing many reactions analogous to those typical of carbenes. Stabilization of R2Si can be achieved by using sufficiently bulky substituents, and electron diffraction data confirm the bent structure of {(Me3Si)2HC}2Si (∠C_Si_C = 970). The sterically demanding 2,4,6-iPr3C6H2 group has been used to stabilize 1.2, the first example of a compound containing conjugated Si=Si bonds. An unusual feature of 1.2 is the preference for the s-cis conformation in both solution and the solid state.
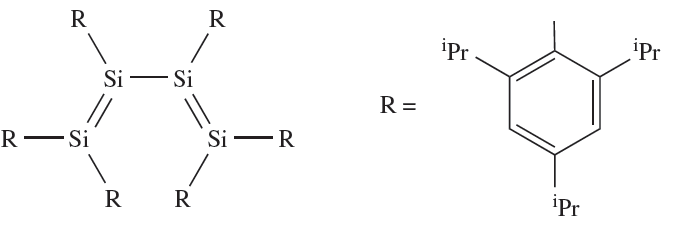
(1.2)



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة إلى كلية مزايا الجامعة للمشاركة في حفل التخرج المركزي الخامس
|
|
|